2023 Reports 5 to 9 of the Auditor General of Canada to the Parliament of Canada
Report 6—Antimicrobial Resistance
At a Glance
Overall, the federal government did not do enough to address the growing resistance to antimicrobial drugs, such as antibiotics, to help safeguard the health of Canadians. The World Health Organization ranks antimicrobial resistance in the top 10 global public health threats, and its effects on the health of Canadians are known. History shows—most recently with the COVID‑19 pandemic—that when it comes to health care, the cost of not being prepared is measured in lives lost.
Addressing antimicrobial resistance in Canada requires coordinated efforts from all levels of government and stakeholders. In June 2023, following the end of our audit period but before our report’s publication, the Public Health Agency of Canada released the Pan‑Canadian Action Plan on Antimicrobial Resistance. We found that the agency worked with the provinces and territories to develop the plan. However, the plan did not cover many important elements—such as concrete deliverables, timelines, and details about who is accountable for each action. Without these key elements, it is unlikely that the plan will result in meaningful actions and produce desired outcomes.
We also found that the Public Health Agency of Canada and Health Canada, who are responsible for improving Canada’s access to new antimicrobials, did not do enough to improve market access to new antimicrobial drugs available in other countries. Both organizations know that successfully encouraging companies to bring new antimicrobials to the Canadian market would require a combination of regulatory and economic incentives.
We found some progress since our last audit on antimicrobial resistance in 2015. For example, in our first audit, we identified that more data on antimicrobial resistance and use was needed to identify trends and support informed decision making. Since then, the Public Health Agency of Canada has improved its data collection. However, some gaps persist, especially around antimicrobial resistance in certain vulnerable populations and in the environment.
Health Canada—as the department responsible for evaluating and monitoring the safety, efficacy, and quality of antimicrobials for use in humans and animals—had strengthened its oversight by implementing regulatory and policy changes to preserve the effectiveness of antimicrobials. For example, the department now requires companies to report annually on the sales of medically important antimicrobials used in food animals. However, we found that the department had not assessed whether the changes it had implemented were working as intended to preserve the effectiveness of antimicrobials.
Key facts and findings
- With nearly 5 million deaths each year associated with antimicrobial resistance worldwide, the World Health Organization referred to antimicrobial resistance as a “silent pandemic” in 2022.
- According to research funded by the Public Health Agency of Canada, it is estimated that 26% of infections in Canada in 2018 did not respond to first-line antimicrobials, with 5,400 deaths attributable to antimicrobial resistance.
- According to the Council of Canadian Academies, by 2050, the rate of resistance to antimicrobials is likely to increase to 40%, with annual deaths in Canada estimated to increase to 13,700.
- Federal funding for antimicrobial-resistance activities other than research was approved for the first time in 2021. Nonetheless, we found that approximately two thirds of funding spent on non-research antimicrobial-resistance activities was pulled from existing budgets in 2021–22 and 2022–23.
- We found that Canadians did not have market access to 19 of the 29 antimicrobial drugs that the World Health Organization classified as reserve antimicrobials, or antimicrobials of last resort.
Why we did this audit
- Antimicrobial resistance already threatens the lives and health of people in Canada and around the world.
- Not only does antimicrobial resistance make existing antimicrobials less effective, but it also forces the health care system to rely on more expensive drugs of last resort, which can also become less effective over time.
- Beyond the human and health care costs, antimicrobial resistance also carries high costs to the economy and Canadians’ livelihoods.
- Reducing antimicrobial resistance in Canada requires a coordinated national response with clear accountabilities, concrete deliverables, specific timelines, and measurable outcomes.
- In recent decades, the development of new antimicrobial drugs worldwide and in Canada has slowed, making it even more important to preserve the effectiveness of available drugs.
Highlights of our recommendations
- The Public Health Agency of Canada, in collaboration with Health Canada, the Canadian Food Inspection Agency, and Agriculture and Agri‑Food Canada, should engage with federal, provincial, and territorial partners and stakeholders to complete, execute, and monitor the Pan Canadian Action Plan on Antimicrobial Resistance.
- Health Canada should finalize its review of veterinary antimicrobials with unspecified or prolonged durations of use and prioritize product label changes by considering, for example, how important these antimicrobials are to human medicine and how often they are sold.
- The Public Health Agency of Canada, in collaboration with Health Canada and federal, provincial, and territorial partners and stakeholders, should use national data on antimicrobial resistance and use to determine which antimicrobials Canadians need most and implement measures to support market access to these drugs.
Please see the full report to read our complete findings, analysis, recommendations and the audited organizations’ responses.


The Public Health Agency of Canada is responsible for collecting data on the United Nations’ Sustainable Development Goal 3 (Good Health and Well-Being) indicator pertaining to the “percentage of bloodstream infections due to selected antimicrobial resistant organisms.”
Visit our Sustainable Development page to learn more about sustainable development and the Office of the Auditor General of CanadaOAG.
Exhibit highlights
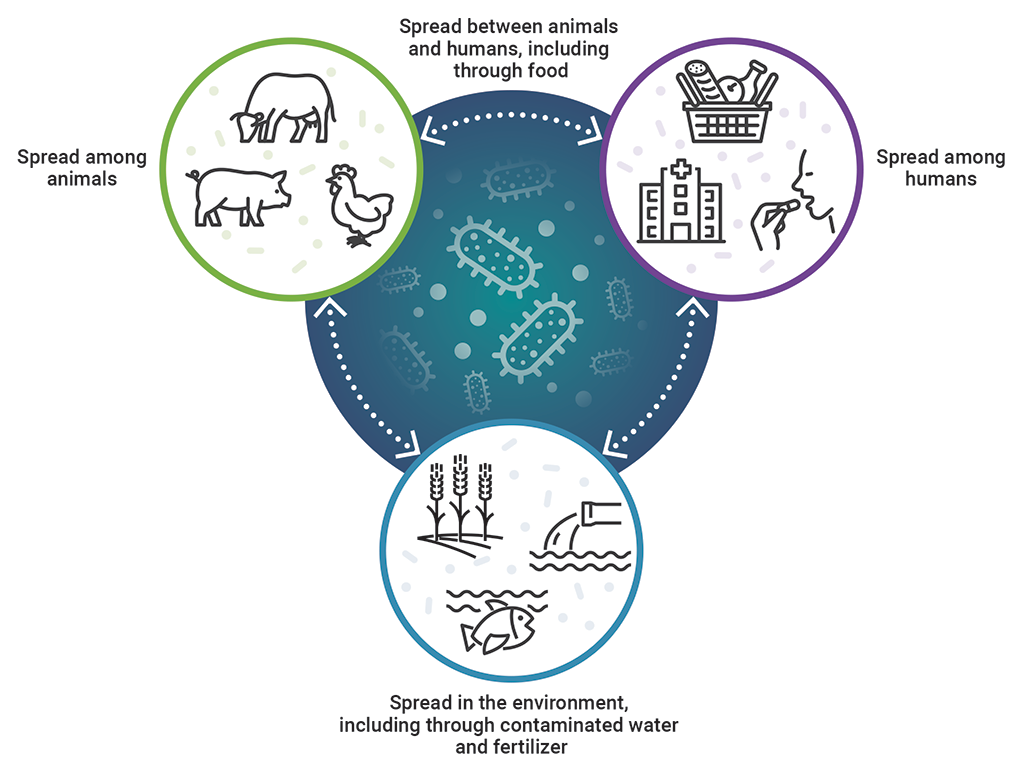
The One Health approach recognizes the interrelationship of humans, animals, and the environment in antimicrobial resistance
Source: Adapted from Tackling Antimicrobial Resistance and Antimicrobial Use: A Pan‑Canadian Framework for Action, Public Health Agency of Canada, 2017
Text version
This image shows the interrelationship of humans, animals, and the environment in antimicrobial resistance, which is recognized by the One Health approach.
Antimicrobial resistance is spread among animals, among humans, and in the environment, including through contaminated water and fertilizer. It is then spread between animals and humans, including through food, and spread between humans, animals, and the environment.
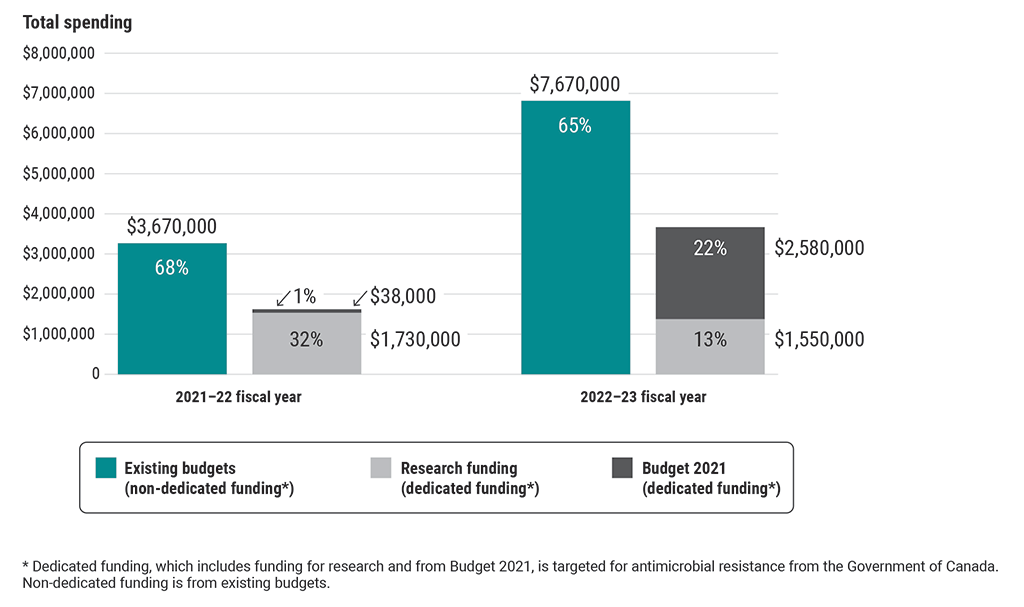
Approximately two thirds of funds spent recently by all 4 federal organizations for antimicrobial-resistance activities examined as part of this audit were from non‑dedicated funding (existing budgets)
Source: Based on estimates from the Public Health Agency of Canada, Health Canada, the Canadian Food Inspection Agency, and Agriculture and Agri‑Food Canada
Text version
This chart shows the types of funding used by all 4 federal organizations for antimicrobial-resistance activities examined as part of this audit in the 2021–22 and 2022–23 fiscal years.
The 3 types of funding spent were non-dedicated funding from existing budgets, dedicated funding for research, and dedicated funding from Budget 2021. Dedicated funding is targeted for antimicrobial-resistance activities from the Government of Canada.
In the 2021–22 fiscal year, the 4 organizations spent $3,670,000 from existing budgets (68%), $1,730,000 of research funding (32%), and $38,000 from Budget 2021 (1%) on antimicrobial-resistance activities.
In the 2022–23 fiscal year, they spent $7,670,000 from existing budgets (65%), $1,550,000 of research funding (13%), and $2,580,000 from Budget 2021 (22%) on antimicrobial-resistance activities.
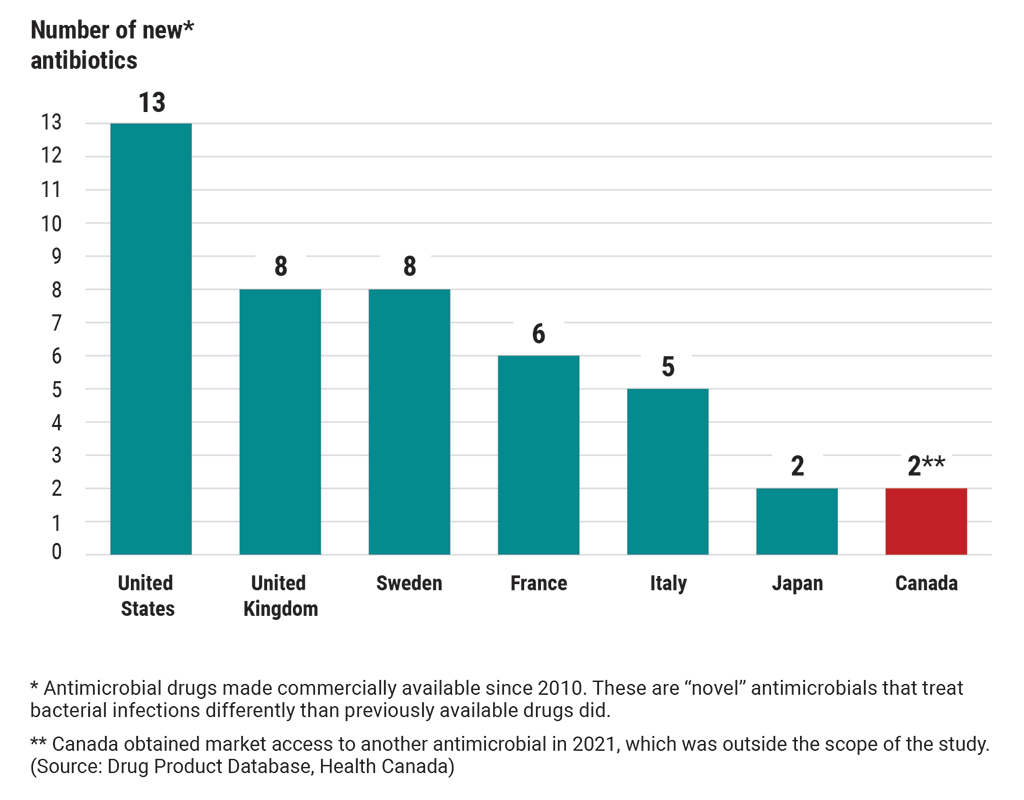
Canada’s access to 13 new antibiotics of last resort lagged behind many other countries as of 2020
Text version
This chart compares Canada’s access to new antibiotics of last resort with 6 other countries’ access to these antibiotics. These are “novel” antibiotics that have been made commercially available since 2010. “Novel” antibiotics treat bacterial infections differently than previously available drugs did.
As of 2020, the United States had access to 13 new antibiotics.
The United Kingdom had access to 8 new antibiotics.
Sweden had access to 8 new antibiotics.
France had access to 6 new antibiotics.
Italy had access to 5 new antibiotics.
Japan had access to 2 new antibiotics.
In 2020, Canada had access to 1 new antibiotic but obtained market access to another antibiotic in 2021, which was outside the scope of the study. (Source: Drug Product Database, Health Canada)
Infographic
Text version
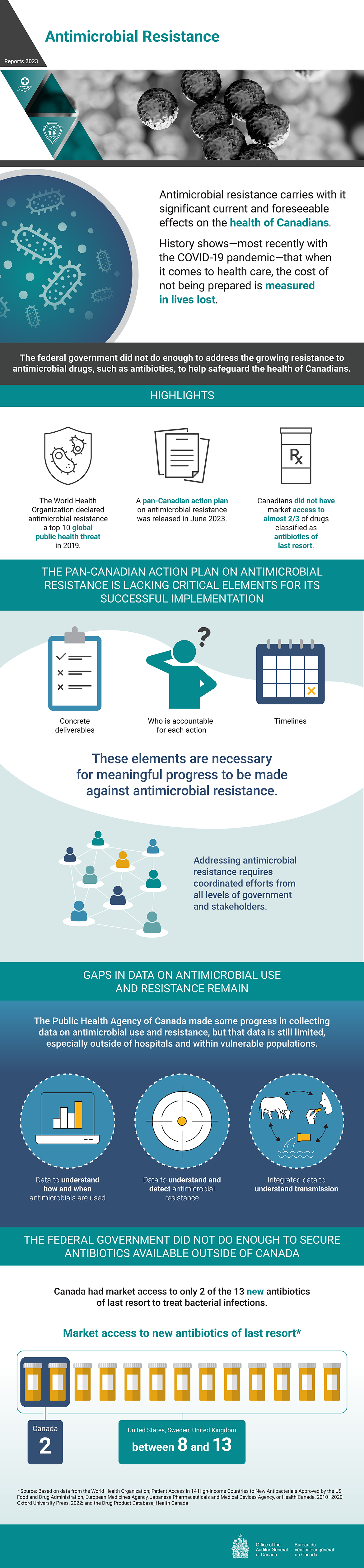
Antimicrobial Resistance
Antimicrobial resistance carries with it significant current and foreseeable effects on the health of Canadians. History shows—most recently with the COVID‑19 pandemic—that when it comes to health care, the cost of not being prepared is measured in lives lost.
The federal government did not do enough to address the growing resistance to antimicrobial drugs, such as antibiotics, to help safeguard the health of Canadians.
Highlights
The World Health Organization declared antimicrobial resistance a top 10 global public health threat in 2019.
A pan-Canadian action plan on antimicrobial resistance was released in June 2023.
Canadians did not have market access to almost two thirds of drugs classified as antibiotics of last resort.
The Pan-Canadian Action Plan on Antimicrobial Resistance is lacking critical elements for its successful implementation
The following elements are necessary for meaningful progress to be made against antimicrobial resistance:
- concrete deliverables
- who is accountable for each action
- timelines
Addressing antimicrobial resistance requires coordinated efforts from all levels of government and stakeholders.
Gaps in data on antimicrobial use and resistance remain
The Public Health Agency of Canada made some progress in collecting data on antimicrobial use and resistance, but that data is still limited, especially outside of hospitals and within vulnerable populations.
Gaps remain in the following 3 types of data on antimicrobial use and resistance:
- data to understand how and when antimicrobials are used
- data to understand and detect antimicrobial resistance
- integrated data to understand transmission
The federal government did not do enough to secure antibiotics available outside of Canada
Canada had market access to only 2 of the 13 new antibiotics of last resort to treat bacterial infections. The United States, Sweden, and the United Kingdom had market access to between 8 and 13.
This market access data is based on data from the World Health Organization; Patient Access in 14 High‑Income Countries to New Antibacterials Approved by the United StatesUS Food and Drug Administration, European Medicines Agency, Japanese Pharmaceuticals and Medical Devices Agency, or Health Canada, 2010–2020, Oxford University Press, 2022; and the Drug Product Database, Health Canada.
Related information
Entities
Tabling date
- 19 October 2023
Related audits
- 2015 Spring Reports of the Auditor General of Canada
Report 1—Antimicrobial Resistance - 2011 Fall Report of the Auditor General of Canada
Chapter 4—Regulating Pharmaceutical Drugs—Health Canada
