2023 Reports 5 to 9 of the Auditor General of Canada to the Parliament of CanadaReport 6—Antimicrobial Resistance
Independent Auditor’s Report
Table of Contents
- Introduction
- Findings and Recommendations
- The pan-Canadian action plan developed under the Public Health Agency of Canada’s leadership was incomplete
- Progress was made to address some gaps in data on antimicrobial resistance and use
- Actions were taken to preserve the effectiveness of antimicrobials, but gaps remain
- The Public Health Agency of Canada and Health Canada had not improved Canada’s market access to antimicrobials
- Conclusion
- About the Audit
- Recommendations and Responses
- Exhibits:
- 6.1—The One Health approach recognizes the interrelationship of humans, animals, and the environment in antimicrobial resistance
- 6.2—Approximately two thirds of funds spent recently by all 4 federal organizations for antimicrobial-resistance activities examined as part of this audit were from non‑dedicated funding (existing budgets)
- 6.3—Canada’s access to 13 new antibiotics of last resort lagged behind many other countries as of 2020
Introduction
Background
6.1 In 2019, the World Health Organization declared antimicrobial resistance as 1 of the top 10 global public health threats. With nearly 5 million deaths each year associated with antimicrobial resistance worldwide, the World Health Organization referred to antimicrobial resistance as a “silent pandemic” in 2022.
6.2 Antimicrobial resistance occurs when pathogens such as bacteria, viruses, and fungi that cause infections in people and animals adapt over time to resist the antimicrobial drugs, such as antibiotics, antivirals, and antifungals, used to treat these infections. The more antimicrobial drugs are used in people and animals, the greater the chance that pathogens develop resistance to these drugs. Some “superbug” pathogens are resistant to multiple drugs.
6.3 Resistant pathogens are found in people and in animals, plants, food, and the environment (for example, in water and soil), which can, in turn, infect humans. Infections caused by resistant pathogens are harder to treat. They also increase the risk of disease spread, severe illness, and death. Furthermore, resistant pathogens can increase the risk posed by medical procedures, such as organ transplantations, joint replacements, and chemotherapy.
6.4 According to research funded by the Public Health Agency of Canada, it was estimated that in 2018, 26% of infections in Canada were resistant to first‑line antimicrobials, with 5,400 deaths attributable to antimicrobial resistance. Furthermore, according to the Council of Canadian Academies, by 2050, the rate of resistance is likely to increase to 40%, resulting in an estimated 13,700 deaths.
6.5 Antimicrobial resistance affects the economy, food production systems, and livelihoods, and it increases costs to the Canadian health care system. The Council of Canadian Academies estimates that in 2018, antimicrobial resistance
- cost Canada $1.4 billion in additional health care spending, because of longer and more complex hospital stays
- reduced Canada’s gross domestic product by $2 billion, including $200 million associated with the animal farming industry, through lower labour productivity
6.6 We acknowledge that the onset of the coronavirus disease (COVID‑19)Definition 1 pandemic significantly affected the operations of the Public Health Agency of Canada and Health Canada. One of the effects was a temporary pause of activities related to the development of a pan‑Canadian action plan on antimicrobial resistance. These activities resumed in late 2021. The pandemic also affected Agriculture and Agri‑Food Canada and the Canadian Food Inspection Agency and other partners and stakeholders that help manage the risks of antimicrobial resistance.
6.7 In Canada, responsibilities related to antimicrobial resistance are shared among the federal, provincial, and territorial governments and with professional organizations, private industry, and non‑governmental organizations. We examined 4 federal organizations that have responsibilities related to antimicrobial resistance:
- Public Health Agency of Canada. This agency is the lead federal organization for combatting antimicrobial resistance and coordinating actions to address the threat of antimicrobial resistance. The agency collects and reports data on antimicrobial-resistant pathogens and antimicrobial use. It raises public awareness, identifies opportunities to improve access to new antimicrobials, and conducts research. The agency also carries out activities to preserve the effectiveness of antimicrobials already being used in Canada—for example, by issuing guidelines on infection prevention and control.
- Health Canada. This department is responsible for promoting and protecting the health and safety of Canadians by regulating health products and food. The department authorizes antimicrobial drugs for sale in Canada for use in humans and animals. This includes continuously evaluating and monitoring the safety, efficacy, and quality of antimicrobials and ensuring that the product monograph and labelling provide adequate directions for use. Health Canada also assesses the risk of food‑borne antimicrobial resistance.
- Canadian Food Inspection Agency. This agency mitigates risks to food safety. This is done to protect Canadians’ health and to maintain a healthy and sustainable animal and plant resource base. This includes regulating medicated livestock feed, which can contain antimicrobial drugs important in human medicine, and supporting Health Canada to ensure these feeds are sold by prescription only. The agency also works with other stakeholders to promote animal health and welfare, including the use of alternatives to antimicrobials such as vaccines and veterinary health products.
- Agriculture and Agri‑Food Canada. This department supports Canada’s agriculture sector. To address antimicrobial resistance, the department promotes collaboration and cooperation across industry and governments and promotes and supports industry action. The department’s research also supports the development of new knowledge to reduce the need to use antimicrobials in agriculture.
Focus of the audit
6.8 This audit focused on whether the Public Health Agency of Canada, Health Canada, the Canadian Food Inspection Agency, and Agriculture and Agri‑Food Canada delivered actions to address antimicrobial resistance, to provide comprehensive surveillance, and to preserve the effectiveness of medically important antimicrobials.
6.9 The audit focused on
- the development of a pan‑Canadian action plan on antimicrobial resistance
- the collection and analysis of data on antimicrobial resistance and use
- efforts to preserve the effectiveness of existing antimicrobials
- efforts to improve access to antimicrobials
6.10 In 2015, we published Report 1—Antimicrobial Resistance, which examined the activities of the Public Health Agency of Canada and Health Canada. The current audit followed up on key findings and recommendations from the 2015 report—specifically, on the collection of antimicrobial resistance and use data, regulatory and policy changes intended to preserve the effectiveness of antimicrobials important in human medicine, and pan‑Canadian efforts led by the Public Health Agency of Canada.
6.11 This audit is important because antimicrobial resistance already threatens the lives and health of people in Canada and around the world. Not only does antimicrobial resistance make existing antimicrobials less effective, but it also forces the health care system to rely on more expensive drugs of last resort, which can also become less effective over time. Beyond the human and health care costs, antimicrobial resistance also carries high costs to the economy and Canadians’ livelihoods.
6.12 More details about the audit objective, scope, approach, and criteria are in About the Audit at the end of this report.
Findings and Recommendations
The pan‑Canadian action plan developed under the Public Health Agency of Canada’s leadership was incomplete
6.13 This finding matters because reducing antimicrobial resistance in Canada requires a coordinated national response with clear accountabilities, concrete deliverables, specific timelines, and measurable outcomes. Without these components, coordinated efforts may be delayed and infections may become harder to treat, thereby increasing risks to Canadians.
6.14 This finding also matters because antimicrobial resistance is a global phenomenon and Canadian efforts contribute to the effectiveness of international efforts.
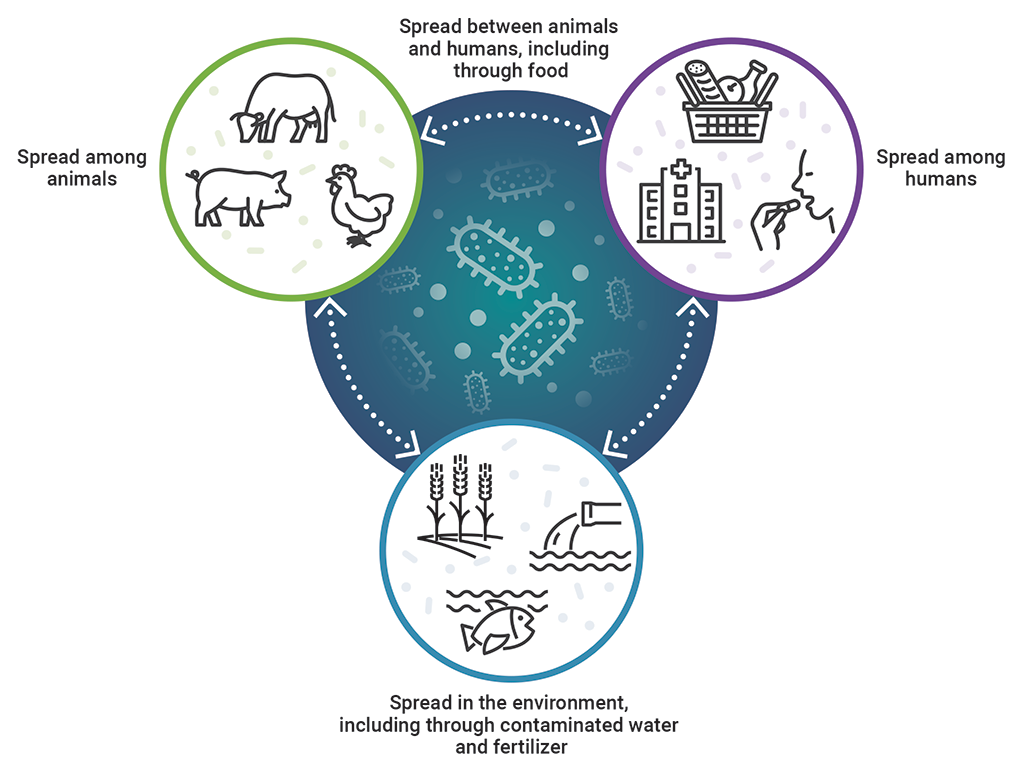
6.15 In May 2015, the World Health Assembly, which Canada is a member of, adopted the World Health Organization’s Global Action Plan on Antimicrobial Resistance. The action plan outlines the One Health approach, which envisions coordinated action across many sectors to balance and optimize the health of people, animals, and the environment (Exhibit 6.1). It also recognizes that antimicrobial resistance is a crisis that must be managed with utmost urgency, and it urges member countries to develop multi‑sectoral national action plans.
Exhibit 6.1—The One Health approach recognizes the interrelationship of humans, animals, and the environment in antimicrobial resistance
Source: Adapted from Tackling Antimicrobial Resistance and Antimicrobial Use: A Pan‑Canadian Framework for Action, Public Health Agency of Canada, 2017
Exhibit 6.1—text version
This image shows the interrelationship of humans, animals, and the environment in antimicrobial resistance, which is recognized by the One Health approach.
Antimicrobial resistance is spread among animals, among humans, and in the environment, including through contaminated water and fertilizer. It is then spread between animals and humans, including through food, and spread between humans, animals, and the environment.
6.16 In 2017, the Public Health Agency of Canada launched Tackling Antimicrobial Resistance and Antimicrobial Use: A Pan‑Canadian Framework as the first phase in Canada’s response to antimicrobial resistance. The framework included a commitment to develop a pan‑Canadian action plan that would
- further define roles and responsibilities for federal, provincial, and territorial governments
- lay out concrete deliverables, timelines, measurable outcomes, and priorities
- allow for the tracking of progress against actions
Lack of specific accountabilities, deliverables, timelines, and measurable outcomes
6.17 The Public Health Agency of Canada led the development of a pan‑Canadian action plan on antimicrobial resistance. The action plan was not completed during our audit period. However, in June 2023, 3 months following the end of the audit period but before our report’s publication, the federal ministers of health and agriculture released the Pan‑Canadian Action Plan on Antimicrobial Resistance. However, we found that the action plan was incomplete.
6.18 We found that the action plan lacked concrete deliverables and timelines. It identified broad roles, responsibilities, and priority actions, but it did not identify which specific federal, provincial, or territorial government organizations were accountable for the actions. We found that desired outcomes, such as an improved understanding of appropriate antimicrobial use, were defined in the action plan but were not measurable. Measurable outcomes are important to track progress of coordinated national actions to reduce antimicrobial resistance in Canada. Without specific accountabilities, deliverables, timelines, and measurable outcomes, there is a risk that action among federal, provincial, and territorial governments to tackle antimicrobial resistance will be delayed, poorly coordinated, and not comprehensive.
6.19 We found that all 4 federal organizations engaged with stakeholders to inform the action plan’s development. Specifically, the Public Health Agency of Canada led the engagement with stakeholders from the human health sector, while all 4 organizations collaborated to engage with the agriculture and animal health sector. We also found that the Public Health Agency of Canada broadened its engagement to include environmental stakeholders, recognizing that this sector is an area of increasing priority. These findings are important because broad stakeholder engagement brings together human, animal, and environmental sectors, which are critical components of the One Health approach.
6.20 We also found that the Public Health Agency of Canada identified the need to supplement the action plan with performance metrics and a federal, provincial, and territorial reporting structure to measure progress. However, there was no target date for completing these activities. Until these key elements are in place, the agency will not be able to demonstrate that Canada is making progress toward meeting its commitments.
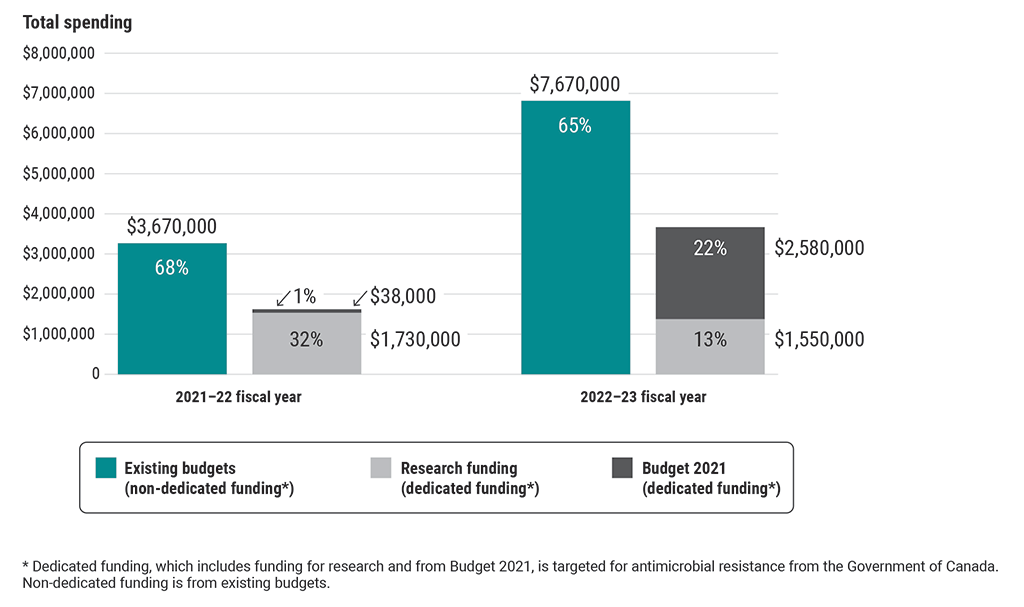
6.21 Prior to 2021, there was no dedicated funding for antimicrobial-resistance activities, except to carry out research (Exhibit 6.2). Without dedicated funding, the 4 organizations were required to identify funds from existing budgets to carry out antimicrobial-resistance activities examined as part of this audit, which represented approximately two thirds of total funds spent on antimicrobial-resistance activities in the 2021–22 and 2022–23 fiscal years. Non‑dedicated funding is subject to competing priorities, which could affect Canada’s sustained ability to deliver on its commitments to combat antimicrobial resistance.
Exhibit 6.2—Approximately two thirds of funds spent recently by all 4 federal organizations for antimicrobial-resistance activities examined as part of this audit were from non‑dedicated funding (existing budgets)
Source: Based on estimates from the Public Health Agency of Canada, Health Canada, the Canadian Food Inspection Agency, and Agriculture and Agri‑Food Canada
Exhibit 6.2—text version
This chart shows the types of funding used by all 4 federal organizations for antimicrobial-resistance activities examined as part of this audit in the 2021–22 and 2022–23 fiscal years.
The 3 types of funding spent were non-dedicated funding from existing budgets, dedicated funding for research, and dedicated funding from Budget 2021. Dedicated funding is targeted for antimicrobial-resistance activities from the Government of Canada.
In the 2021–22 fiscal year, the 4 organizations spent $3,670,000 from existing budgets (68%), $1,730,000 of research funding (32%), and $38,000 from Budget 2021 (1%) on antimicrobial-resistance activities.
In the 2022–23 fiscal year, they spent $7,670,000 from existing budgets (65%), $1,550,000 of research funding (13%), and $2,580,000 from Budget 2021 (22%) on antimicrobial-resistance activities.
6.22 The first dedicated funding for non‑research activities was included in Budget 2021, when the Public Health Agency of Canada, Health Canada, and the Canadian Food Inspection Agency received approval for $28.6 million over 5 years with $5.7 million per year thereafter. This included support for data collection on antimicrobial resistance and use and the development of a national list of reserve antimicrobials, or antimicrobials of last resort. Budget 2023 identified funding for the Public Health Agency of Canada to help secure new antimicrobial drugs for Canadians. However, Budget 2023 did not include specific funding for the action plan’s implementation.
6.23 Using estimates provided by all 4 federal organizations, a total of $34.6 million was spent in the 2021–22 and 2022–23 fiscal years on activities to address antimicrobial resistance, including research. Of this amount, just over an estimated $17.2 million was spent on activities in the scope of the audit.
6.24 The Public Health Agency of Canada, in collaboration with Health Canada, the Canadian Food Inspection Agency, and Agriculture and Agri‑Food Canada, should engage with federal, provincial, and territorial partners and stakeholders to complete, execute, and monitor the Pan‑Canadian Action Plan on Antimicrobial Resistance. These actions should include
- measurable outcomes, concrete deliverables, timelines, performance metrics, and the monitoring of progress
- an identification of accountable federal, provincial, and territorial partners for specific outcomes and deliverables
- an assessment of the funding and resources needed to successfully execute the action plan
Response of each entity. Agreed.
See Recommendations and Responses at the end of this report for detailed responses.
Progress was made to address some gaps in data on antimicrobial resistance and use
6.25 This finding matters because collecting, analyzing, and reporting data on antimicrobial resistance allows emerging resistant infections to be detected and trends in antimicrobial use to be identified. This information can support timely public health interventions and inform effective health programs, guidelines, and policies.
6.26 Antimicrobial resistance affects some population groups differently. For example, it is known to be more prevalent among people who are at greater risk of infection, such as people in congregate living and long‑term care and those with weaker immune systems, including the elderly.
6.27 The Public Health Agency of Canada is responsible for leading national efforts to continuously and systematically collect, analyze, and interpret data on antimicrobial resistance and use in Canada. The agency carries out this responsibility in collaboration with the provinces and territories and by operating 11 programs that collect data on drug‑resistant organisms and antimicrobial use on farms, in hospitals, and to some extent in the community (that is, outside of hospitals). Information from these programs is publicly reported annually by the Public Health Agency of Canada in the Canadian Antimicrobial Resistance Surveillance System report.
6.28 The Public Health Agency of Canada is also responsible for collecting data on the United Nations’ Sustainable Development Goal 3 (Good Health and Well‑Being) indicator pertaining to the “percentage of bloodstream infections due to selected antimicrobial-resistant organisms.”
6.29 In our 2015 audit, we found that the agency had identified weaknesses in its collection of information on antimicrobial resistance and use outside of hospitals and in specific vulnerable populations, such as in Indigenous peoples. We also noted the need for the agency to collect more information on how and why antimicrobials were being used in animals and the need for timelier reporting of the information it collected and analyzed.
Data collected on antimicrobial resistance has increased, but more is needed
6.30 We found that the Public Health Agency of Canada allocated approximately $14.6 million, or just over 50% of its 5‑year funding approved through Budget 2021, to improve the collection and analysis of antimicrobial resistance and use data.
6.31 We found that the agency launched initiatives to help close gaps pertaining to the collection of data outside of hospitals and in vulnerable populations. The agency had increased data collected on antimicrobial-resistant infections outside of hospitals using laboratory data from Ontario, Saskatchewan, and Prince Edward Island, which represent approximately 40% of the Canadian population. We also found that the agency had collected disaggregated data on vulnerable populations through its program for antimicrobial-resistant gonorrhea and had taken steps to expand this program.
6.32 We found that, despite these improvements, other initiatives to collect antimicrobial resistance and use data outside of hospitals and in vulnerable populations were in development but had missed early milestones. We found that the Public Health Agency of Canada did not have all the data it needed to report on the United Nations’ Sustainable Development Goal indicator related to bloodstream infections. We also found that the agency had identified other data needs, including the need to detect antimicrobial resistance and use trends in other vulnerable populations and in the environment. However, this work was not funded.
6.33 All 4 federal organizations had also undertaken actions to better understand the use of medically important antimicrobials (antimicrobials that are also used to treat serious infections in humans) in food animals. We found that
- Health Canada and the Public Health Agency of Canada collected data on the volume of medically important antimicrobials sold for use in food animals
- the Public Health Agency of Canada expanded its collection of information on reasons for antimicrobial use from selected farms across the country
- the Canadian Food Inspection Agency and Agriculture and Agri‑Food Canada provided some funding for a system that had started to collect data on antimicrobials prescribed by veterinarians
6.34 In addition, we found that the Public Health Agency of Canada did not provide data to the World Health Organization for its report on global antimicrobial resistance for 2020. The agency provided the organization with Canadian data from 2020 for the 2021 reporting cycle. We also found that the agency did not systematically collect resistance data on 1 of the 8 pathogens tracked by the World Health Organization because the agency had not assessed it as a high‑priority pathogen and because related infections were not commonly reported in Canada. However, an increase in extensively drug‑resistant infections caused by this pathogen had recently been reported in the United States and Europe. The prevalence of this resistant pathogen in Canada is not well understood because of the lack of monitoring. We found that the agency last reviewed its list of priority pathogens in 2015. As of spring 2023, the agency was considering different approaches to reviewing and updating the list.
6.35 We found that the agency had publicly reported against its targets for reducing specific antimicrobial-resistant infections and for reducing resistance to a medically important antimicrobial found in retail meat samples. However, we also found that the agency had not developed performance indicators and targets to assess whether its data collection, analysis, and reporting activities led to informed public health interventions, such as strategies to improve antimicrobial use or guidelines to prevent and control antimicrobial-resistant infections.
6.36 The Public Health Agency of Canada should close the remaining identified gaps in its collection, analysis, and reporting of antimicrobial resistance and use data by prioritizing these gaps based on risks to public health, commitments in the Pan‑Canadian Action Plan on Antimicrobial Resistance, available resources, and national and international reporting commitments.
The agency’s response. Agreed.
See Recommendations and Responses at the end of this report for detailed responses.
Actions were taken to preserve the effectiveness of antimicrobials, but gaps remain
6.37 This finding matters because in recent decades, the development of new antimicrobial drugs worldwide and in Canada has slowed, making it even more important to preserve the effectiveness of available drugs. This requires a set of coordinated actions to promote prudent antimicrobial use in humans and animals (that is, not using a drug at all or using the right drug at the right dose, frequency, and duration) and to improve, monitor, and evaluate antimicrobial use.
6.38 According to the Public Health Agency of Canada, there are 22 times more animals than people in Canada. Food animals account for approximately 80% of medically important antimicrobials used in Canada. Medically important antimicrobials are those antimicrobials used in veterinary medicine that are also important for treating infections in humans. While antimicrobials are important for treating and preventing infections in humans and food animals, imprudent use can lead to the development of antimicrobial-resistant pathogens that can spread among humans, animals, and their shared environment.
6.39 In our 2015 audit, we found that Health Canada needed to strengthen control over the importation of veterinary antimicrobial drugs and to periodically review the use of medically important antimicrobials in food animals so that such use did not pose undue risks to human health.
6.40 We also observed that the Public Health Agency of Canada and Health Canada had taken some steps to promote prudent antimicrobial use in humans. However, we noted the importance of continued work in this area.
Gaps in preserving the effectiveness of antimicrobials used in humans
6.41 We found that Health Canada and the Public Health Agency of Canada had expanded efforts to preserve the effectiveness of antimicrobials used in humans. However, the Public Health Agency of Canada, in collaboration with Health Canada, had not finalized an approach to coordinate federal, provincial, and territorial activities to preserve the effectiveness of antimicrobials. This is important so that federal, provincial, and territorial efforts are not duplicated, reach the greatest number of individuals, and are tailored where possible for vulnerable populations.
6.42 The World Health Organization classifies antibiotics, a type of antimicrobial, under the “access, watch, and reserve” (AWaRe) system. This classification provides guidance on which antibiotics should be used first (access), which antibiotics should be used more cautiously (watch), and which antibiotics should be used only as a last resort (reserve). We found that Health Canada received funding through Budget 2021 to develop a national list of reserve antimicrobials for humans. Although Health Canada’s work did not include “access” and “watch” classifications, it is a first step toward classifying antimicrobials important to human medicine. We found that Health Canada had consulted with experts on a draft list of reserve antimicrobials and planned further consultations with the public by August 2023, with finalization and publication to follow. According to Health Canada, the United Kingdom and Australia have adopted classification systems similar to AWaRe.
6.43 We also found that the Public Health Agency of Canada had not developed national prescribing guidelines for the use of antimicrobials in human medicine. Subsequent to our audit period, the Pan‑Canadian Action Plan on Antimicrobial Resistance identified the Public Health Agency of Canada as being responsible for leading the development, review, endorsement, dissemination, and promotion of national prescribing guidelines for human medicine. However, timelines for completion were not included. These guidelines are important to support a coordinated pan‑Canadian approach that includes the national reserve list so that the effectiveness of existing antimicrobials is preserved.
6.44 We found that the Public Health Agency of Canada and Health Canada worked to raise public awareness about the importance of prudent antimicrobial use. For example, the Public Health Agency of Canada had initiatives to preserve the effectiveness of antimicrobials for human use, such as the Do Bugs Need Drugs education campaign. Another example was activities in support of World Antimicrobial Awareness Week, led by the agency in collaboration with Health Canada.
6.45 We found that the Public Health Agency of Canada did not assess the effectiveness of any of its initiatives to determine whether they had improved awareness of antimicrobial resistance and the importance of prudent antimicrobial use. As a result, Health Canada could not benefit from such an assessment as it moved forward with its own efforts to develop educational materials to be provided to patients on the importance of the prudent use of antimicrobials.
6.46 We also found that Health Canada had launched an initiative to include statements on the labels of antimicrobials for human use that encouraged the prudent prescribing and use of these drugs. We reviewed a representative sample of 32 product labels and found that all of the labels included such statements.
6.47 The Public Health Agency of Canada and Health Canada should work together, and with federal, provincial, and territorial partners and stakeholders, to develop an approach to guide federal, provincial, and territorial efforts to preserve the effectiveness of antimicrobials that includes national prescribing guidelines and an expanded classification of antimicrobials for human use. The approach should include defined accountabilities, concrete deliverables, measurable outcomes, and timelines.
Response of each entity. Agreed.
See Recommendations and Responses at the end of this report for detailed responses.
Several improvements in regulatory and policy oversight of antimicrobials used in animals
6.48 We found that Health Canada had implemented several regulatory and policy changes to help close gaps related to the use of antimicrobials in food animals, some of which were identified in our 2015 audit report. Health Canada did the following:
- required veterinary prescriptions for medically important antimicrobials used in animals, including those used in medicated animal feed
- removed growth-promotion claims from the labels of medically important antimicrobials used in animals
- strengthened control over the importation of medically important antimicrobials for use in food animals
- required pharmaceutical companies to report annually to Health Canada sales of medically important antimicrobials for use in food animals
- developed a framework to periodically review the use of medically important antimicrobials in food animals to minimize risks to human health
6.49 However, we found that Health Canada had not assessed the effects of some of these changes. Health Canada had been requiring the annual submission of medically important antimicrobial sales data from pharmaceutical companies since 2018 but had not worked with partners to use this data to establish baselines or to set measurable goals for reducing use. Also, Health Canada and the Canadian Food Inspection Agency had not assessed whether their joint approach was sufficient to verify that feed mills and retail feed stores complied with the requirement to mix and sell medicated feed containing medically important antimicrobials by prescription only.
6.50 Health Canada monitors medically important antimicrobials used in food animals so that the use of these antimicrobials does not contribute to antimicrobial resistance in humans. We found that Health Canada was reviewing the labels of just over 100 products containing medically important antimicrobials. At the time these products were approved, their labelling allowed for prolonged or undefined periods of use, which can contribute to antimicrobial resistance. Revising these product labels is important because these antimicrobial products are among the most commonly sold and because their labelling should specify limiting use to the treatment and prevention of disease and recommend avoiding long or unspecified periods of use. We found that the department had not prioritized the revision of product labels by considering, for example, how important these antimicrobials are to human medicine and how often they are sold.
6.51 To help prioritize its review activities, the department categorized antimicrobials by importance to human medicine. This categorization is also used by the agricultural industry, animal health professionals, and federal partners to assist in priority setting, to support veterinary education programs, to inform decisions about antimicrobial use, and to facilitate the analysis and reporting of antimicrobial resistance and use data. We found that Health Canada had not updated its categorization document since 2009 and that the document did not include all of the antimicrobials classified by the World Health Organization as being important to human medicine. This is important because an updated categorization document would better inform decisions on the regulation and prudent use of antimicrobials in animals.
6.52 Health Canada should review and revise, as necessary, its joint agreement with the Canadian Food Inspection Agency to determine whether it effectively identifies and corrects instances of non‑compliance with the requirement that feed mills and retail feed stores sell feed containing medically important antimicrobials by prescription only.
Response of each entity. Agreed.
See Recommendations and Responses at the end of this report for detailed responses.
6.53 Health Canada, the Public Health Agency of Canada, the Canadian Food Inspection Agency, and Agriculture and Agri‑Food Canada, in collaboration with partners and stakeholders, should use antimicrobial sales data alongside other antimicrobial resistance and use data, where necessary, to establish appropriate baselines for use and measurable goals for reducing antimicrobial use.
Response of each entity. Agreed.
See Recommendations and Responses at the end of this report for detailed responses.
6.54 Health Canada should finalize its review of veterinary antimicrobials with unspecified or prolonged durations of use and prioritize product label changes by considering, for example, how important these antimicrobials are to human medicine and how often they are sold.
The department’s response. Agreed.
See Recommendations and Responses at the end of this report for detailed responses.
6.55 Health Canada should update, as needed, its categorization of medically important antimicrobials to inform its priority setting so that stakeholders have the most current information to support the development of veterinary education programs and inform decisions about antimicrobial use.
The department’s response. Agreed.
See Recommendations and Responses at the end of this report for detailed responses.
The Public Health Agency of Canada and Health Canada had not improved Canada’s market access to antimicrobials
6.56 This finding matters because antimicrobials become less effective over time. According to the Council of Canadian Academies, it is estimated that in 2018, 26% of infections in Canada did not respond to first‑line antimicrobials and that this may grow to 40% by the year 2050. Furthermore, the Public Health Agency of Canada estimated that approximately a third of high‑risk resistant pathogens in Canada could be treated with antimicrobials available in other countries but not in Canada.
6.57 The development and commercialization of new antimicrobials have faced challenges worldwide. This is partly because antimicrobials are less profitable to develop and market than other drugs because they should be used sparingly for short durations. Antimicrobials also become less effective as pathogens develop resistance to them. To offset these challenges and encourage companies to bring new antimicrobials to market, several countries have launched initiatives, such as economic incentives.
6.58 According to the Public Health Agency of Canada, these challenges are compounded in Canada by the country’s small market, regulatory environment, and approach to drug pricing. In 2015, the Public Health Agency of Canada, in collaboration with federal partners, recognized the need for Canadians to have increased market access to new antimicrobials.
Insufficient action to improve market access to antimicrobial drugs available in other countries
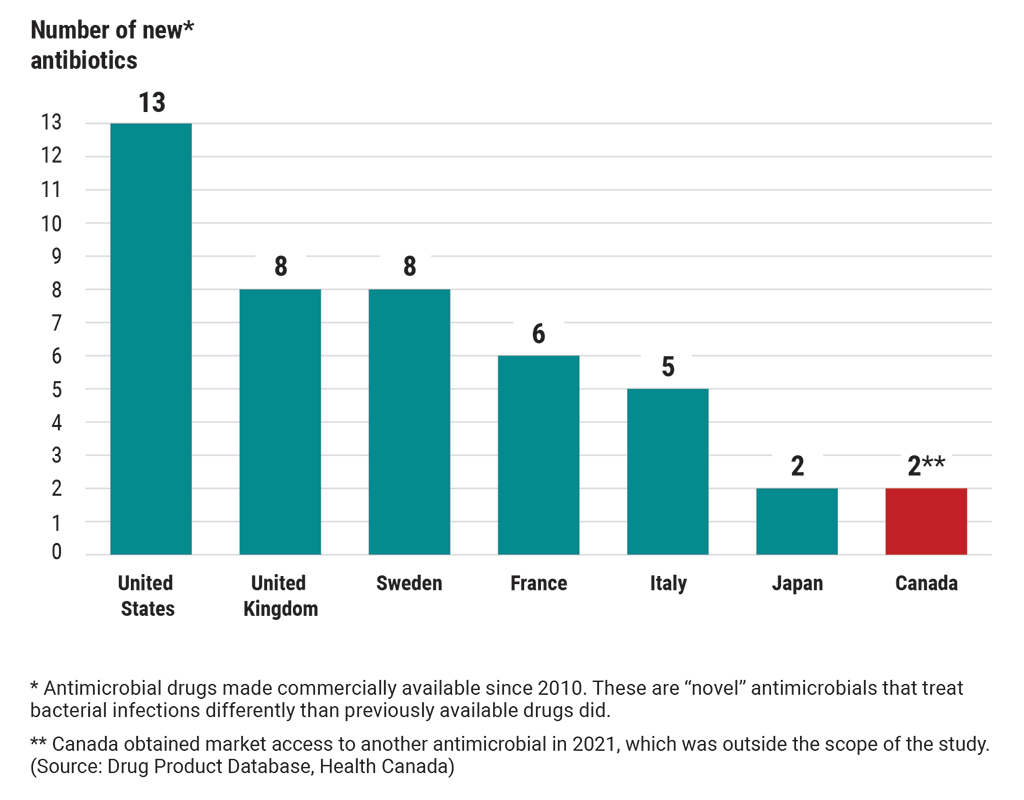
6.59 We found that Canadians did not have market access to 19 of the 29 antibiotics that the World Health Organization had classified as reserve antibiotics, or antibiotics of last resort. These antimicrobials are critical to treating multi‑drug‑resistant infections. Of the 29 reserve antibiotics, 13 were newly developed drugs that treat bacterial infections in different ways. Other countries, such as the United States, Sweden, and the United Kingdom, had market access to between 8 and 13 of these drugs. As of 2020, Canadians had market access to 1 of these drugs. Canadians obtained market access to another of these drugs in 2021 (Exhibit 6.3).
Exhibit 6.3—Canada’s access to 13 new antibiotics of last resort lagged behind many other countries as of 2020
Source: Based on data from the World Health Organization; Patient Access in 14 High‑Income Countries to New Antibacterials Approved by the US Food and Drug Administration, European Medicines Agency, Japanese Pharmaceuticals and Medical Devices Agency, or Health Canada, 2010–2020, Oxford University Press, 2022; and the Drug Product Database, Health Canada
Exhibit 6.3—text version
This chart compares Canada’s access to new antibiotics of last resort with 6 other countries’ access to these antibiotics. These are “novel” antibiotics that have been made commercially available since 2010. “Novel” antibiotics treat bacterial infections differently than previously available drugs did.
As of 2020, the United States had access to 13 new antibiotics.
The United Kingdom had access to 8 new antibiotics.
Sweden had access to 8 new antibiotics.
France had access to 6 new antibiotics.
Italy had access to 5 new antibiotics.
Japan had access to 2 new antibiotics.
In 2020, Canada had access to 1 new antibiotic but obtained market access to another antibiotic in 2021, which was outside the scope of the study. (Source: Drug Product Database, Health Canada)
6.60 Canadian health professionals can access drugs not available on the Canadian market through Health Canada’s Special Access Programme if a patient has a serious or life‑threatening condition for which existing treatments have failed or are unsuitable and the manufacturer agrees to provide the drug. As noted in Exhibit 6.3, Canada lacks market access to 11 of 13 new reserve antibiotics. We found that out of these 11 antibiotics, 9 had been requested through the programme between 2012 and 2022. One of these drugs was requested 78 times in 2022 and an additional 29 times between January and March 2023. In our view, this demonstrates that some demand exists for these drugs in Canada.
6.61 We found that since 2016, Health Canada and the Public Health Agency of Canada had examined Canada’s lack of market access to new antimicrobial drugs but had yet to take effective actions to improve the situation. In addition, both organizations have known that successfully encouraging companies to bring new antimicrobials to the Canadian market would require a combination of regulatory and economic incentives.
6.62 We also found that Health Canada implemented a regulatory incentive in 2018 to accelerate the review of antimicrobial drugs that treat serious life‑threatening infections that had no or limited treatment options available in Canada. The department found that the incentive did not significantly increase new antimicrobial drug submissions. However, it did not pursue other regulatory incentives, such as reducing fees for the review of antimicrobial drug submissions.
6.63 We found that the Public Health Agency of Canada had recently started to examine economic incentives that could address some of the challenges associated with bringing antimicrobials available in other countries to the Canadian market while also allowing these drugs to be used sparingly. One example of an economic incentive is guaranteeing companies a minimum level of revenue regardless of sales.
6.64 The Public Health Agency of Canada, in collaboration with Health Canada and federal, provincial, and territorial partners and stakeholders, should use national data on antimicrobial resistance and use to determine which antimicrobials Canadians need most and implement measures to support market access to these drugs.
Response of each entity. Agreed.
See Recommendations and Responses at the end of this report for detailed responses.
Conclusion
6.65 We concluded that the Public Health Agency of Canada, Health Canada, the Canadian Food Inspection Agency, and Agriculture and Agri‑Food Canada did not do enough to deliver actions to address antimicrobial resistance, to provide comprehensive surveillance, and to preserve the effectiveness of medically important antimicrobials.
About the Audit
This independent assurance report was prepared by the Office of the Auditor General of Canada on the actions taken by the Public Health Agency of Canada, Health Canada, the Canadian Food Inspection Agency, and Agriculture and Agri‑Food Canada to address antimicrobial resistance. Our responsibility was to provide objective information, advice, and assurance to assist Parliament in its scrutiny of the government’s management of resources and programs and to conclude on whether the actions taken to address antimicrobial resistance complied in all significant respects with the applicable criteria.
All work in this audit was performed to a reasonable level of assurance in accordance with the Canadian Standard on Assurance Engagements (CSAE) 3001—Direct Engagements, set out by the Chartered Professional Accountants of Canada (CPA Canada) in the CPA Canada Handbook—Assurance.
The Office of the Auditor General of Canada applies the Canadian Standard on Quality Management 1—Quality Management for Firms That Perform Audits or Reviews of Financial Statements, or Other Assurance or Related Services Engagements. This standard requires our office to design, implement, and operate a system of quality management, including policies or procedures regarding compliance with ethical requirements, professional standards, and applicable legal and regulatory requirements.
In conducting the audit work, we complied with the independence and other ethical requirements of the relevant rules of professional conduct applicable to the practice of public accounting in Canada, which are founded on fundamental principles of integrity, objectivity, professional competence and due care, confidentiality, and professional behaviour.
In accordance with our regular audit process, we obtained the following from entity management:
- confirmation of management’s responsibility for the subject under audit
- acknowledgement of the suitability of the criteria used in the audit
- confirmation that all known information that has been requested, or that could affect the findings or audit conclusion, has been provided
- confirmation that the audit report is factually accurate
Audit objective
The objective of this audit was to determine whether the Public Health Agency of Canada, Health Canada, the Canadian Food Inspection Agency, and Agriculture and Agri‑Food Canada delivered actions to address antimicrobial resistance, to provide comprehensive surveillance, and to preserve the effectiveness of medically important antimicrobials.
Scope and approach
This audit examined the efforts by the Public Health Agency of Canada, Health Canada, the Canadian Food Inspection Agency, and Agriculture and Agri‑Food Canada to combat antimicrobial resistance in Canada.
Specifically, we examined the Public Health Agency of Canada’s leadership role in developing a pan‑Canadian action plan on antimicrobial resistance. This included determining whether the action plan outlined accountabilities, deliverables, measurable outcomes, and timelines. We also examined whether the Public Health Agency of Canada, in collaboration with Health Canada, the Canadian Food Inspection Agency, and Agriculture and Agri‑Food Canada, identified and addressed known surveillance gaps for antimicrobial resistance and use, including those highlighted in our 2015 audit report on antimicrobial resistance. Surveillance refers to data on antimicrobial resistance and use.
We examined whether the Public Health Agency of Canada, Health Canada, the Canadian Food Inspection Agency, and Agriculture and Agri‑Food Canada delivered on their responsibilities and commitments to preserve the effectiveness of antimicrobials in human medicine, which includes medically important antimicrobials that are also used in food animals. We also examined whether the Public Health Agency of Canada and Health Canada had improved Canadians’ access to antimicrobial medicines.
The audit team also considered gender-based analysis plus and the United Nations’ Sustainable Development Goal 3 (Good Health and Well‑Being), including indicator 3.d.2 (the percentage of bloodstream infections due to selected antimicrobial-resistant organisms).
The audit methodology included interviews with federal organization officials and stakeholders, document reviews, data sampling, and analysis. Where representative sampling was used, samples were sufficient in size to conclude on the sampled population with a confidence level of no less than 90% and a margin of error (confidence interval) of no greater than +10%.
The audit did not examine
- animal health and welfare
- alternatives to antimicrobials
- the regulation of antimicrobials in disinfectants or pesticides
- antimicrobial use in companion animals
- the effectiveness of the antimicrobials available in Canada
Criteria
We used the following criteria to conclude against our audit objective:
| Criteria | Sources |
|---|---|
|
The Public Health Agency of Canada has developed, in collaboration with partners, a pan‑Canadian action plan for antimicrobial resistance that includes concrete accountabilities, deliverables, outcomes, and timelines. |
|
|
The Public Health Agency of Canada, in collaboration with the Canadian Food Inspection Agency, Health Canada, and Agriculture and Agri‑Food Canada, has identified and addressed gaps in its surveillance of antimicrobial resistance and use. |
|
|
The Public Health Agency of Canada, Health Canada, the Canadian Food Inspection Agency, and Agriculture and Agri‑Food Canada have delivered on their responsibilities and commitments to preserve the effectiveness of medically important antimicrobials. |
|
Period covered by the audit
The audit covered the period from 1 April 2021 to 31 March 2023. This is the period to which the audit conclusion applies. However, to gain a more complete understanding of the subject matter of the audit, we also examined certain matters that preceded the start date of this period. We also considered the Pan‑Canadian Action Plan on Antimicrobial Resistance, which was released by the Public Health Agency of Canada in June 2023, as a subsequent event.
Date of the report
We obtained sufficient and appropriate audit evidence on which to base our conclusion on 29 September 2023, in Ottawa, Canada.
Audit team
This audit was completed by a multidisciplinary team from across the Office of the Auditor General of Canada led by Markirit Armutlu, Principal. The principal has overall responsibility for audit quality, including conducting the audit in accordance with professional standards, applicable legal and regulatory requirements, and the office’s policies and system of quality management.
Recommendations and Responses
In the following table, the paragraph number preceding the recommendation indicates the location of the recommendation in the report.
| Recommendation | Response |
|---|---|
|
6.24 The Public Health Agency of Canada, in collaboration with Health Canada, the Canadian Food Inspection Agency, and Agriculture and Agri‑Food Canada, should engage with federal, provincial, and territorial partners and stakeholders to complete, execute, and monitor the Pan‑Canadian Action Plan on Antimicrobial Resistance. These actions should include
|
The Public Health Agency of Canada’s response. Agreed. The Public Health Agency of Canada has intensified its collaboration with Health Canada, the Canadian Food Inspection Agency, Agriculture and Agri‑Food Canada, and other partners to advance actions to implement the Pan‑Canadian Action Plan on Antimicrobial Resistance, building on the published compendium to the action plan, which maps progress and activities already underway or planned, across federal, provincial, and territorial governments that support the action plan’s 10 shared priority actions. Beginning in October 2023, the Public Health Agency of Canada, with Health Canada, the Canadian Food Inspection Agency, and Agriculture and Agri‑Food Canada, will engage other federal, provincial, and territorial partners, as appropriate, to establish measurable outcomes, deliverables, timelines, and accountabilities, as well as an approach for monitoring and reporting on progress (to be completed by September 2024). The Public Health Agency of Canada, in collaboration with its partners, will assess the federal resources required to support implementation of the Pan‑Canadian Action Plan on Antimicrobial Resistance, respecting jurisdictional roles and responsibilities by December 2025. This work will include identifying and prioritizing gaps, assessing implementation risks, and identifying mitigation measures and alternative implementation approaches. Health Canada’s response. Agreed. Health Canada will support the Public Health Agency of Canada as the federal lead and will work with partners and stakeholders to complete, execute, and monitor the Pan‑Canadian Action Plan on Antimicrobial Resistance. The Canadian Food Inspection Agency’s response. Agreed. The Canadian Food Inspection Agency will support the Public Health Agency of Canada and work with other partners and stakeholders to complete, execute, and monitor the Pan‑Canadian Action Plan on Antimicrobial Resistance. Agriculture and Agri‑Food Canada’s response. Agreed. Within the context of its mandate focused on the development of a competitive, innovative, and sustainable agriculture and agri‑food sector, Agriculture and Agri‑Food Canada is supportive of the recommendation and will continue to engage with implicated parties in the agriculture space (that is, provinces and territories, industry partners, and stakeholders), where appropriate, to advance and promote a coordinated and collaborative approach to support the implementation of the Pan‑Canadian Action Plan on Antimicrobial Resistance. |
|
6.36 The Public Health Agency of Canada should close the remaining identified gaps in its collection, analysis, and reporting of antimicrobial resistance and use data by prioritizing these gaps based on risks to public health, commitments in the Pan‑Canadian Action Plan on Antimicrobial Resistance, available resources, and national and international reporting commitments. |
The Public Health Agency of Canada’s response. Agreed. The Public Health Agency of Canada, in collaboration with provincial and territorial partners, as outlined in the Pan‑Canadian Action Plan on Antimicrobial Resistance, will close the remaining gaps in its collection, analysis, and reporting of antimicrobial resistance and antimicrobial use data. The assessment and prioritization of antimicrobial resistance and antimicrobial use gaps will be completed by March 2025. The agency will expand sources, coverage, and integration of antimicrobial resistance and antimicrobial use surveillance data, including the use of modern laboratory technologies and standardized reporting, to help monitor antimicrobial resistance and antimicrobial use across One Health sectors with a focus on improving data from the environment; transmission pathways between sectors; and populations disproportionately impacted by antimicrobial resistance and inappropriate antimicrobial use. To help accomplish this, the agency will build on investments from Budget 2021 to enhance collection of antimicrobial resistance and antimicrobial use surveillance data from the community sector (such as primary care and long‑term care data) targeting completion by December 2025. The agency will also enhance data on vulnerable populations and integrate provincial and territorial antimicrobial resistance laboratory data into domestic and international reporting. These activities will help inform timely and effective public health interventions, programs, guidelines, and policies. |
|
6.47 The Public Health Agency of Canada and Health Canada should work together, and with federal, provincial, and territorial partners and stakeholders, to develop an approach to guide federal, provincial, and territorial efforts to preserve the effectiveness of antimicrobials that includes national prescribing guidelines and an expanded classification of antimicrobials for human use. The approach should include defined accountabilities, concrete deliverables, measurable outcomes, and timelines. |
The Public Health Agency of Canada’s response. Agreed. The Public Health Agency of Canada will engage with federal, provincial, and territorial partners, key subject matter experts, and stakeholders to develop an agreed‑upon approach to guide efforts to preserve the effectiveness of antimicrobials. This approach, to be completed by September 2024, will include clear accountabilities and measures of progress and focus on supporting education and awareness activities and develop and promote national prescribing guidelines. This work will be ongoing and iterative to ensure that new knowledge is considered. Following implementation, the outcomes of these activities will be assessed annually through monitoring of prescribing practices in various Canadian health care settings, through a Canadian antimicrobial prescribing survey and antimicrobial sales/procurements and/or use, using third‑party data, noting that measuring behaviour change requires monitoring over the longer term. Efforts with clinical and scientific subject matter experts are already underway to develop evidence-based national antimicrobial prescribing guidelines and increase their accessibility for health professionals using advanced information-technology solutions, expected to be available by December 2024. The Public Health Agency of Canada will also support the development of an expanded classification of antimicrobials for human use, building upon Health Canada’s work to develop the Canadian list of reserve antimicrobials for human use. Health Canada’s response. Agreed. Health Canada will finalize its work to develop the Canadian list of reserve antimicrobials for human use. Health Canada will develop an expanded Canadian classification of antimicrobial drugs for human use with the support of the Public Health Agency of Canada and other partners by the end of the 2026–27 fiscal year. Health Canada will also support the Public Health Agency of Canada and collaborate with federal, provincial, and territorial partners and stakeholders to develop an approach to guide federal, provincial, and territorial efforts to preserve the effectiveness of antimicrobials for human use, in line with its roles and responsibilities as a regulatory authority to promote the responsible use of antimicrobial drugs approved for human use on the Canadian market. |
|
6.52 Health Canada should review and revise, as necessary, its joint agreement with the Canadian Food Inspection Agency to determine whether it effectively identifies and corrects instances of non‑compliance with the requirement that feed mills and retail feed stores sell feed containing medically important antimicrobials by prescription only. |
Health Canada’s response. Agreed. Health Canada will work in collaboration with the Canadian Food Inspection Agency to review and revise, as necessary, its joint agreement by the end of the 2024–25 fiscal year. The review will focus on assessing the joint approach to verify compliance of commercial feed mills and outlets mixing and selling feed containing medically important antimicrobials by prescription only. The Canadian Food Inspection Agency’s response. Agreed. The Canadian Food Inspection Agency will assess the joint approach by Health Canada and the agency related to ensuring livestock feeds containing medically important antimicrobials are only sold to purchasers with a veterinary prescription. Plans are being made for Health Canada and the agency to assess the approach by the end of the 2024–25 fiscal year. The agency will analyze the resource costs associated with collecting information for Health Canada, will analyze whether the agency continues to have the capacity to collect the information, and will consider the impacts of continuing to conduct versus ceasing the activity. The agency and Health Canada will assess if the resources being used lead to improved compliance amongst regulated parties. The assessment will also analyze the methods of communication between the 2 organizations to ensure a mutually beneficial structure that will support future reporting. |
|
6.53 Health Canada, the Public Health Agency of Canada, the Canadian Food Inspection Agency, and Agriculture and Agri‑Food Canada, in collaboration with partners and stakeholders, should use antimicrobial sales data alongside other antimicrobial resistance and use data, where necessary, to establish appropriate baselines for use and measurable goals for reducing antimicrobial use. |
Health Canada’s response. Agreed. Health Canada will fulfill its role in collaborating with federal, provincial, and territorial governments, partners, and stakeholders to work toward establishing baselines and measurable goals to promote improved antimicrobial stewardship, protect human and animal health, and preserve the effectiveness of medically important antimicrobials. Health Canada, as the administrator of the Veterinary Antimicrobial Sales Reporting system, will work with data providers to support the provision of good quality data required by March 31 of each year to ensure the data is compliant with the annual reporting requirements. Health Canada will share with federal partners and stakeholders the annual data collected under the system in the third quarter of each year so that it can be used to inform measures of progress. The Public Health Agency of Canada’s response. Agreed. The Public Health Agency of Canada will work with Health Canada, the Canadian Food Inspection Agency and Agriculture and Agri‑Food Canada, in collaboration with provincial and territorial governments, partners, and stakeholders, toward establishing baselines and measurable goals to promote improved antimicrobial stewardship, to protect human and animal health, and to preserve the effectiveness of medically important antimicrobials. This collective work will include developing appropriate science-based methodologies for establishing baselines and measurable goals, and identifying jurisdictional roles and responsibilities, timelines, and resource requirements. Because of differences in food animal production, their diseases, and how they are raised, approaches and timelines may vary by sector. In 2024 and ongoing, the Public Health Agency of Canada will support federal discussion on this recommendation as part of the implementation of the Pan‑Canadian Action Plan on Antimicrobial Resistance and participate in stakeholder engagement. The agency will continue to analyze surveillance data from the Veterinary Antimicrobial Sales Reporting system (joint surveillance activity led by Health Canada and the agency), which, along with data on antimicrobial resistance in animals, food, and humans and antimicrobial use collected by the Canadian Integrated Program for Antimicrobial Resistance Surveillance system (led by the agency), will contribute to informing the baselines and goals. The Canadian Food Inspection Agency’s response. Agreed. The Canadian Food Inspection Agency will fulfill its role in collaborating with partners and stakeholders in establishing baselines and measurable goals for reductions of antimicrobial use. Veterinarians are a critical component related to prudent antimicrobial use. The agency will develop and deliver educational material aimed at veterinarians related to medicated livestock feed use by the end of the 2025–26 fiscal year. Throughout the implementation of the 5‑year Pan‑Canadian Action Plan on Antimicrobial Resistance (2023–2028), the agency will maintain effective and collaborative relationships with its partners and stakeholders to advance actions to reduce antimicrobial use and develop methods to measure the reductions in livestock production. Agriculture and Agri‑Food Canada’s response. Agreed. Agriculture and Agri‑Food Canada, in tandem with relevant departments and agencies, will build on existing antimicrobial sales and use data to engage with provinces and territories, industry partners, and stakeholders in the work leading to the development of appropriate baselines, goals, and measures of progress for reductions in antimicrobial use in animal agriculture. This activity is supported and identified in the Pan‑Canadian Action Plan on Antimicrobial Resistance. Starting in 2024, collaborative discussions on the implementation of the Pan‑Canadian Action Plan on Antimicrobial Resistance between governments and industry partners will include identifying approaches and resource requirements toward establishing appropriate baselines for use and measurable goals for reducing antimicrobial use. |
|
6.54 Health Canada should finalize its review of veterinary antimicrobials with unspecified or prolonged durations of use and prioritize product label changes by considering, for example, how important these antimicrobials are to human medicine and how often they are sold. |
Health Canada’s response. Agreed. Health Canada is committed to advancing work on the responsible use of antimicrobials in animals while still maintaining access to safe and efficacious antimicrobials on the Canadian market. As this work requires an iterative process with stakeholder consultations and active participation from veterinarians, producer associations, and the pharmaceutical industry, finalizing the review of all products will be dependent on required evidence and the complexity of issues associated with the products. Health Canada has completed its preliminary review of products that may require labelling updates and will build on this work using a stepwise approach, as per the published re‑evaluation framework, to review and update the labels of veterinary antimicrobials that are important in human medicine. Prioritization and the first review of product label updates will occur over the 5‑year time frame of the Pan‑Canadian Action Plan on Antimicrobial Resistance to be completed by the end of the 2027 fiscal year. |
|
6.55 Health Canada should update, as needed, its categorization of medically important antimicrobials to inform its priority setting so that stakeholders have the most current information to support the development of veterinary education programs and inform decisions about antimicrobial use. |
Health Canada’s response. Agreed. Health Canada will update, as needed, its categorization document of antimicrobial drugs based on importance in human medicine, to assist with the pre- and post‑market evaluation of veterinary antimicrobials, priority setting, and stewardship activities by the end of the 2025 calendar year. Health Canada will consult with subject matter experts and stakeholders when revising this document to reflect the most current information available. |
|
6.64 The Public Health Agency of Canada, in collaboration with Health Canada and federal, provincial, and territorial partners and stakeholders, should use national data on antimicrobial resistance and use to determine which antimicrobials Canadians need most and implement measures to support market access to these drugs. |
The Public Health Agency of Canada’s response. Agreed. The Public Health Agency of Canada, in coordination with Health Canada and other partners, will determine which new antimicrobials are of the highest priority to gain market access in Canada, using national and other available data on antimicrobial resistance and use by March 2024. The agency will also work with partners to implement measures to support sustained market access to antimicrobial drugs for Canadians, subject to the agency’s mandate, roles, and responsibilities of other relevant federal and national institutions. Health Canada’s response. Health Canada will collaborate with the Public Health Agency of Canada and federal, provincial, and territorial partners and stakeholders to determine which antimicrobials Canadians need most and to implement measures to support market access to these drugs for human health in line with its responsibilities as a regulatory authority. |

