2022 Reports 9 and 10 of the Auditor General of Canada to the Parliament of CanadaReport 9—COVID-19 Vaccines
Independent Auditor’s Report
Table of Contents
- Introduction
- Findings and Recommendations
- Procurement and authorization of COVID‑19 vaccines
- Allocation and distribution of vaccines
- Surveillance of vaccines
- Conclusion
- About the Audit
- Recommendations and Responses
- Exhibits:
Introduction
Background
9.1 Vaccines are considered to be one of the most important public health tools available for preventing serious illness and controlling infectious disease outbreaks. The rapidly evolving nature of the coronavirus disease (COVID‑19)Definition 1 pandemic, which was declared in March 2020, and the high demand across the world for vaccines put pressure on the federal government to act quickly on COVID‑19 vaccine approval, procurement, distribution, and surveillance.
9.2 Canada’s COVID‑19 Immunization Plan: Saving Lives and Livelihoods, established by the federal, provincial, and territorial governments in December 2020, has the goal of enabling as many Canadians as possible to be immunized against COVID‑19 as quickly as possible while ensuring that high-risk populations are prioritized.
9.3 In Canada, COVID‑19 vaccine approval and immunization rollout began in December 2020 for adults, in May 2021 for adolescents, in November 2021 for children aged 5 years to 11 years, and in July 2022 for children aged 6 months to 5 years.
9.4 In Canada, public health is a shared responsibility between the federal government, the provinces, and the territories. Vaccine distribution logistics are usually provincial and territorial responsibilities. During the COVID‑19 pandemic, the federal government paid for the vaccines and paid for and managed the distribution logistics for vaccine delivery to the provinces and territories. Canada’s immunization response to COVID‑19 involved collaboration between the Government of Canada, the provincial and territorial governments, Indigenous organizations, municipal governments, public health and logistical experts, vaccine companies, and Canadians.
9.5 Provincial and territorial governments. These governments are responsible for prioritizing vaccine distribution by population and managing initial and subsequent doses within their jurisdictions, guided by advice provided by the federal government. The provinces and territories are also responsible for requesting the quantity of doses and types of vaccines needed to immunize their populations and providing the federal government with coverage and safety information on vaccine use within these populations.
9.6 Public Health Agency of Canada. The agency is the lead federal organization facilitating national approaches to public health in cooperation with provincial and territorial health departments or agencies. In relation to COVID‑19 vaccines, the agency is responsible for identifying vaccine needs, paying vaccine companies, allocating and distributing vaccines to the provinces and territories, and conducting surveillance of vaccines.
9.7 Health Canada. The department is responsible for authorizing vaccines before they can be made available in Canada. Before authorizing a particular vaccine, the department evaluates whether the vaccine’s benefits outweigh the potential risks. The department also continues to monitor the safety and effectiveness of vaccines after they are released for use.
9.8 Public Services and Procurement Canada. The department purchases goods and services for federal departments and agencies. It was responsible for leading negotiations with COVID‑19 vaccine companies and putting in place agreements with the companies on behalf of the Public Health Agency of Canada.
Focus of the audit
9.9 This audit focused on whether
- Public Services and Procurement Canada provided adequate procurement support to secure COVID‑19 vaccines
- the Public Health Agency of Canada and Health Canada efficiently provided access to COVID‑19 vaccines
- the Public Health Agency of Canada and Health Canada’s surveillance of COVID‑19 vaccines was effective and timely
9.10 This audit is important because factors such as global travel, urbanization, and climate change are making health emergencies like the COVID‑19 pandemic more likely to occur. Widespread immunization is one of the best tools for protecting people against infectious diseases. To reduce deaths, hospitalizations, and negative effects on Canadians’ health and social and economic well-being during future health emergencies, it is important to learn from this pandemic and to be better prepared for future public health emergencies.
9.11 Although we had access to the advance purchase agreementsDefinition 2 established to purchase the COVID‑19 vaccines and to the related documentation, we are limited in what we can report because of confidentiality clauses in the agreements. In addition, we did not audit any science-based decisions.
9.12 More details about the audit objective, scope, approach, and criteria are in About the Audit at the end of this report.
Findings and Recommendations
Overall message
9.13 Overall, the Public Health Agency of Canada and Health Canada, supported by Public Services and Procurement Canada, responded to the urgent nature of a rapidly evolving coronavirus pandemic by working together to obtain a sufficient number of COVID‑19 vaccine doses for provinces and territories to vaccinate everyone living in Canada. At the end of May 2022, the agency reported that about 82% of people who were eligible to be vaccinated at that time had received at least 2 doses. This was the largest mass vaccination program in Canadian history.
9.14 Although the government was successful in sending a sufficient number of doses to provinces and territories, the Public Health Agency of Canada ended up with a large surplus of doses. This led to vaccine wastage because some of the doses expired before they could be used or donated. At the end of May 2022, there were 32.5 million doses in inventory, and using unclassified and public documentation, we estimated those doses to be worth about $1 billion. The majority of those doses will expire by the end of 2022, resulting in more wastage if they are not used or donated soon. Canada, like other countries, is trying to donate some of its surplus. In addition, there were delays in implementing the information technology system that the Public Health Agency of Canada acquired to track the distribution and use of vaccine doses, necessitating workarounds for inventory management. Not all of the system’s functionalities were being used by the end of our audit.
9.15 Because of known problems that were not addressed before the pandemic, sharing case-level details of COVID‑19 vaccine surveillance data was not as effective as it should have been. When the pandemic began in early 2020, the Public Health Agency of Canada did not have regulations or finalized agreements with the provinces and territories to clearly outline what public health surveillance information to share and how to share it. By the end of our audit period, the agency had not received permission from all provinces and territories to share detailed case-level information on vaccine safety with Health Canada, vaccine companies, and the World Health Organization. Data sharing will continue to be important as new vaccines are developed and manufactured, including in Canada, to keep Canadians safe.
Procurement and authorization of COVID‑19 vaccines
Public Services and Procurement Canada secured a sufficient supply of vaccine doses for Canada
9.16 We found that Public Services and Procurement Canada provided efficient procurement support to the Public Health Agency of Canada. Soon after Canada’s COVID‑19 Vaccine Task Force made recommendations regarding potential vaccines, Public Services and Procurement Canada secured a sufficient supply of COVID‑19 vaccine doses to meet the country’s needs.
9.17 The analysis supporting this finding discusses the following topics:
9.18 This finding matters because vaccines were needed quickly to reduce Canadians’ risk of serious illness, hospitalization, and death from COVID‑19. Despite the urgency to acquire vaccine doses, vaccine procurement needed to be done with due diligence.
9.19 At the start of the pandemic, there was uncertainty about which COVID‑19 vaccines would be developed and authorized and when the vaccines would be available for distribution. This uncertainty created high global demand for safe and effective vaccines across the world and put pressure on governments to secure agreements with vaccine companies. The uncertainty around vaccine supply made the market very competitive. In such an environment, advance payments and obligations for minimum purchase were required. Furthermore, Canada had very limited domestic capacity to produce vaccines and therefore was reliant on international imported products.
9.20 The federal government established the COVID‑19 Vaccine Task Force, which was made up of science and industry experts who provided recommendations on identifying and selecting vaccines. The task force had its first meeting on 16 June 2020 and provided its first letter of advice on 29 June 2020.
Efficient procurement support
9.21 We found that Public Services and Procurement Canada provided efficient procurement support to the Public Health Agency of Canada to obtain COVID‑19 vaccines. Public Services and Procurement Canada modified its procurement processes early in the pandemic to allow use of its emergency contracting authority for vaccine procurement. The authority allowed the department to procure vaccines with the vaccine companies recommended by the COVID‑19 Vaccine Task Force using a non-competitive approach.
9.22 Public Services and Procurement Canada started negotiating with vaccine companies after the recommendations from the COVID‑19 Vaccine Task Force were received in late June 2020. On behalf of the Public Health Agency of Canada and with its approval, Public Services and Procurement Canada established advance purchase agreements with 7 vaccine companies that demonstrated the potential to develop viable vaccines, with the goal of securing various types of COVID‑19 vaccines and enough doses to fully vaccinate every eligible person in Canada.
9.23 We found that, although a non-competitive approach was taken, Public Services and Procurement Canada exercised due diligence on the 7 vaccine companies by conducting assessments to examine the companies’ financial capability to meet requirements and by conducting integrity checks to mitigate the risk of unethical business practices. We found no issue with the delegation of authority because the Minister of Public Services and Procurement signed the 7 advance purchase agreements.
Sufficient vaccine doses obtained
9.24 By 19 January 2021, Public Services and Procurement Canada had put in place 7 advance purchase agreements for up to 414 million potential doses. These agreements included advance payments required to support vaccine development, testing, and at-risk manufacturing. The strategy was to secure agreements with several vaccine companies in case Health Canada authorized only 1 or a few vaccines. The agreements reached were with the following companies (listed in chronological order):
- Moderna—24 July 2020
- Sanofi—11 September 2020
- Pfizer—26 October 2020
- Medicago—13 November 2020
- AstraZeneca—21 November 2020
- Johnson & Johnson—30 November 2020
- Novavax—19 January 2021
The department also worked with vaccine companies to secure deliveries as quickly as possible. Procurement of the following additional vaccine doses took place to respond to many emerging factors, including delayed deliveries and the need for boosters:
- In February 2021, a contract with Verity Pharmaceuticals Canada IncorporatedInc. / Serum Institute of India was signed to bring 2 million AstraZeneca vaccine doses rapidly to Canada.
- In March 2021, approximately 5 million additional AstraZeneca doses were procured while there were delays in getting doses from other vaccine companies.
- In April and August 2021, 2 amendments were put in place with Moderna and Pfizer for up to 280 million additional doses over 3 years (2022–24).
- The department secured vaccines from Pfizer, Moderna, Novavax, and Medicago for 2022 and 2023, with options to extend into 2024. For Sanofi doses, the deliveries would be confirmed if vaccine authorization is received.
9.25 On 27 July 2021, the Government of Canada announced that Canada had received more than 66 million doses of COVID‑19 vaccines. This meant that there were enough doses (2 doses each) to fully vaccinate every person in Canada who was eligible at that time (people aged 12 years and older). The federal government’s target to provide vaccines to those who wanted them by fall 2021 was met.
9.26 In order to protect the commercial confidential information contained in the advance purchase agreements, we used publicly available information and unclassified information to estimate that at 31 May 2022, the average cost of 1 dose was approximately $30, excluding taxes. The estimated cost per dose will vary over time based on a number of factors, including, but not limited to, the effects of changes in foreign currency exchange rates and in market forces, such as supply and demand. As a result, at the end of our audit period, the Government of Canada had spent approximately $5 billion on vaccines for the 169 million doses paid for between December 2020 and May 2022 (see Exhibit 9.3, after paragraph 9.54).
Health Canada expedited the authorization of vaccines
9.27 We found that Health Canada adapted its normal authorization process into one that expedited the regulatory approval to authorize COVID‑19 vaccines before they were made available in Canada. We found that the expedited process included the same key steps as the normal process to authorize vaccines, but it was adjusted to allow for a rolling submission across the review period.
9.28 The analysis supporting this finding discusses the following topic:
9.29 This finding matters because, despite the urgent need for a COVID‑19 vaccine, an expedited vaccine authorization process required the same level of diligence as the normal vaccine authorization process.
Expedited process to authorize vaccines
9.30 We found that Health Canada followed a systematic process to authorize the COVID‑19 vaccines used in Canada. (We did not audit the science-based decisions on vaccine authorization.) Because of the urgent need for COVID‑19 vaccines, the department adapted its normal review process to expedite the vaccine authorization process. The expedited process, put in place through an interim order, allowed vaccine companies to submit partially completed applications, called rolling submissions, at the time of the initial filing with Health Canada. Health Canada began its assessment using the initial information and accepted new evidence as it became available until the application was deemed complete. This is a different approach than the normal Food and Drug Regulations process to authorize drugs, which requires all information to be provided at the time of submission.
9.31 We compared the steps for authorizing COVID‑19 vaccines in the expedited process with the Food and Drug Regulations process. We found that Health Canada followed the requirements in the regulations for reviewing scientific information even if it was not all received at the same time. For example, we found that Health Canada completed clinical and quality evaluations of data received from vaccine companies.
9.32 The department had a dedicated team of scientists who reviewed the vaccine applications, and we also saw evidence of reviews completed by supervisors. In addition, guidance, standard operating procedures, and information sessions were available to employees who were conducting the regulatory review work. The interim order provided the authority to impose terms and conditions on any authorization. We found that the vaccines were authorized with terms and conditions that included requirements for the vaccine companies to provide safety, effectiveness, and quality information to support Health Canada’s efforts to monitor the safety of COVID‑19 vaccines. (See Exhibit 9.1 for the timing of a selection of vaccine authorizations.)
Exhibit 9.1—Health Canada expedited its processes for vaccine authorization during the COVID‑19 pandemicNote *
|
11 March 2020 |
The World Health Organization declares the global outbreak of COVID‑19 to be a pandemic. |
|
16 September 2020 |
Interim Order Respecting the Importation, Sale and Advertising of Drugs for Use in Relation to COVID‑19. |
|
1 October 2020 |
Initial submission from AstraZeneca is received by Health Canada. |
|
9 October 2020 |
Initial submission from Pfizer is received by Health Canada. |
|
12 October 2020 |
Initial submission from Moderna is received by Health Canada. |
|
30 November 2020 |
Initial submission from Johnson & Johnson is received by Health Canada. |
|
9 December 2020 |
The Pfizer vaccine for people aged 16 years and older is authorized by Health Canada. |
|
23 December 2020 |
The Moderna vaccine for people aged 18 years and older is authorized by Health Canada. |
|
29 January 2021 |
Initial submission from Novavax is received by Health Canada. |
|
26 February 2021 |
The AstraZeneca vaccine for people aged 18 years and older is authorized by Health Canada. The AstraZeneca vaccine for people aged 18 years and older that is manufactured by the Serum Institute of India is authorized by Health Canada. (This authorization expired on 16 September 2021.) |
|
5 March 2021 |
The Johnson & Johnson vaccine for people aged 18 years and older is authorized by Health Canada. |
|
18 March 2021 |
The Regulations Amending the Food and Drug Regulations is to include the COVID‑19 expedited process in the Food and Drug Regulations. |
|
19 April 2021 |
Initial submission from Medicago is received by Health Canada. |
|
5 May 2021 |
The Pfizer vaccine for people aged 12 years and older is authorized by Health Canada. |
|
27 August 2021 |
The Moderna vaccine for people aged 12 years and older is authorized by Health Canada. |
|
19 November 2021 |
The Pfizer vaccine for people aged 5 years and older is authorized by Health Canada. |
|
17 February 2022 |
The Novavax vaccine for people aged 18 years and older is authorized by Health Canada. |
|
24 February 2022 |
The Medicago vaccine for people aged 18 years to 64 years is authorized by Health Canada. |
|
17 March 2022 |
The Moderna vaccine for people aged 6 years to 11 years is authorized by Health Canada. |
|
Source: World Health Organization and various federal government sources |
|
9.33 Health Canada stated that, although expedited, the process used for COVID‑19 vaccines respected the Food and Drug Regulations standards for vaccine review (safety, efficacy, and quality). In March 2021, the regulations were amended to include the expedited process for COVID‑19 vaccines.
Allocation and distribution of vaccines
9.34 Implementing an immunization program across Canada that ensured equitable allocation and distributionDefinition 3 and that prioritized high-risk populations required collaboration and planning. Canada’s immunization response involved collaboration between the Government of Canada and other parties, including provinces, territories, representatives from Indigenous groups, logistical experts, and vaccine companies.
9.35 Canada’s National Advisory Committee on Immunization is made up of experts from across Canada in the fields of immunization, infectious diseases, epidemiology, and other fields and has provided guidance on the use of vaccines since 1964. In relation to COVID‑19 vaccines, the committee provided guidance to the Public Health Agency of Canada on the prioritization of populations for early immunization. The committee also made recommendations to support decision making for COVID‑19 immunization at the provincial and territorial levels. The committee considers specific factors when providing advice, such as economics, ethics, equity, feasibility, and acceptability. It identified the following high-risk populations:
- those at high risk of severe illness and death from COVID‑19
- those most likely to transmit COVID‑19 to those at high risk of severe illness and death from COVID‑19 and workers essential to maintaining the COVID‑19 response
- those contributing to the maintenance of other essential services for the functioning of society
- those whose living or working conditions put them at elevated risk of infection and those for which infection could have disproportionate consequences, including Indigenous communities
9.36 During the pandemic, for the first time, the Public Health Agency of Canada assumed the lead role of managing the distribution of vaccines from companies to the provinces and territories. This included managing logistical requirements for the different vaccines, including availability, timing, warehousing, packaging, shipping, and cold chain maintenance (that is, maintaining the vaccines at specific temperatures during storage and shipping).
The Public Health Agency of Canada equitably allocated vaccine doses to provinces and territories and distributed requested doses in a timely way
9.37 We found that the Public Health Agency of Canada equitably allocated COVID‑19 vaccine doses to the provinces and territories while prioritizing high-risk populations. We also found that the agency delivered requested doses to provinces and territories in a timely way.
9.38 The analysis supporting this finding discusses the following topics:
9.39 This finding matters because it was crucial that provinces and territories receive COVID‑19 vaccine doses in order to immunize their populations as quickly as possible. In addition, prioritizing the allocation of vaccines to high-risk populations is essential to reduce severe outcomes, such as hospitalizations and deaths.
Equitable allocation of vaccine doses
9.40 We found that the Public Health Agency of Canada equitably allocated vaccine doses to the provinces and territories, as the federal, provincial, and territorial governments had agreed in December 2020. The equitable allocation agreement required that vaccines be allocated on a per capita basis, taking into consideration the size of high-risk populations identified by the National Advisory Committee on Immunization. Provinces and territories requested shipments of vaccine doses from the amounts that they were allocated. We found that the agency delivered all vaccine doses requested by the provinces and territories (Exhibit 9.2). In addition, we found that federal, provincial, territorial, and Indigenous representatives participated in working groups, advisory committees, and steering committees throughout the audit period, which allowed for the consideration of different views before decisions were reached.
Exhibit 9.2—At the end of May 2022, the Public Health Agency of Canada had delivered all the vaccine doses requested by provinces and territories
| Provincial or territorial recipient | Provincial or territorial population size (in thousands) |
Total cumulative doses allocated (in thousands) |
Total doses requested by the province or territory and delivered by the agency (in thousands) |
|---|---|---|---|
| Ontario | 15,008 | 36,836 | 36,688 |
| Quebec | 8,653 | 23,148 | 22,703 |
| British Columbia | 5,287 | 13,404 | 12,619 |
| Alberta | 4,501 | 11,697 | 11,521 |
| Manitoba | 1,393 | 3,775 | 3,748 |
| Saskatchewan | 1,186 | 3,160 | 3,089 |
| Nova Scotia | 1,007 | 2,624 | 2,491 |
| New Brunswick | 800 | 2,181 | 2,175 |
| Newfoundland and Labrador | 523 | 1,498 | 1,484 |
| Prince Edward Island | 168 | 440 | 425 |
| Northwest Territories | 46 | 119 | 116 |
| Yukon | 43 | 107 | 103 |
| Nunavut | 40 | 109 | 108 |
| Total | 38,655 | 99,098Note * | 97,270 |
|
Source: Public Health Agency of Canada and Statistics Canada |
|||
9.41 We found that, although high-risk populations were considered in vaccine allocation, the agency relied on the National Advisory Committee on Immunization’s prioritization of populations. The agency did not conduct its own gender-based analysis plusDefinition 4 as required in its internal policy and to meet federal government commitments.
Timely distribution to provinces and territories
9.42 We found that the Public Health Agency of Canada distributed the requested number of COVID‑19 vaccine doses to provinces and territories in a timely way. From the first vaccine delivery in December 2020 to the end of May 2022, the vaccines were delivered on average 2 days after a province or territory requested an order. In our view, deliveries were timely, given the range of logistical requirements faced by the agency to successfully deliver the vaccines.
9.43 We also noted instances in which the agency responded quickly when provinces and territories faced problems with vaccine deliveries. For example, when vaccines arrived damaged, the agency arranged for another shipment. In addition, when provinces and territories requested doses above their respective allocations to meet emerging needs, we noted instances in which the agency liaised with the other provinces and territories when needed to help find the required doses.
9.44 The agency contracted logistics providers FedEx Express Canada Corporation and Innomar Strategies Inc. for vaccine delivery and storage. This contributed to the agency’s ability to deliver vaccine doses in a timely way. These providers were primarily responsible for providing centralized and safe warehousing and timely distribution of COVID‑19 vaccines to provinces and territories (including to remote and isolated communities).
The Public Health Agency of Canada was unsuccessful in its efforts to minimize vaccine wastage
9.45 We found that the Public Health Agency of Canada was unsuccessful in its efforts to minimize vaccine wastage. The agency had a surplus of vaccine doses resulting from the obligation to buy doses as specified in advance purchase agreements and the purchase of optional doses. We also found that minimizing wastage was affected by the agency’s delay in implementing important functionalities of VaccineConnect, the information technology system intended to support planning and managing the COVID‑19 vaccines and decision making around vaccine wastage.
9.46 The analysis supporting this finding discusses the following topics:
- Unsuccessful efforts to minimize vaccine wastage
- Delays in implementing a vaccine management information technology system
9.47 This finding matters because vaccine wastage results in a financial cost to Canadians.
9.48 When advance purchase agreements were signed with the 7 vaccine companies starting in July 2020, it was unknown which vaccines would be approved for use in Canada or when vaccines would be available for distribution. Based on the market realities at the time, these agreements contained binding requirements to purchase a specific number of doses, and options were included to buy additional doses. The agreements ranged from 20 to 76 million total doses. By signing several advance purchase agreements, there were more chances to obtain the sufficient number of vaccines for Canada’s needs, but there was also the possibility that, should all vaccines receive Health Canada’s authorization, Canada would have a surplus of vaccine doses.
9.49 In June 2021, the federal government publicly committed to donating doses to other countries that needed vaccines. Other countries with a surplus of vaccines had also committed to making donations.
9.50 Vaccines have expiry dates that need to be considered when allocating, distributing, and donating doses. For example, it is not possible to donate vaccine doses when their expiry dates have been extended because the labels would not show these extensions. Wastage can happen for many reasons. For example, wastage can occur when surplus doses expire or when there is ineffective temperature control. Addressing supply management problems requires collaboration between different federal organizations and stakeholders, including the Public Health Agency of Canada, Public Services and Procurement Canada, and in the case of international donations, Global Affairs Canada.
Unsuccessful efforts to minimize vaccine wastage
9.51 The Public Health Agency of Canada knew that the potential existed for the advance purchase agreements to result in excess doses and identified this as a risk in summer 2020. To mitigate the risk, the agency expected to donate excess doses to other countries and to work with vaccine companies to manage the vaccine supply.
9.52 As at 31 May 2022, 6 of 7 potential vaccines had been authorized for use in Canada, resulting in an oversupply of vaccine doses. With each authorized vaccine came an obligation to purchase a specific quantity of doses and, for all but 1 agreement, options to purchase additional doses.
9.53 We found that the agency purchased optional doses from Pfizer and Moderna, further contributing to the oversupply. This led to vaccine wastage as some of the doses expired before they could be used or donated. Officials explained that, because of the evolving nature of the pandemic, there was a need to keep buying optional doses, to expand coverage, to accelerate deliveries, and to address waning immunity and changes in vaccine administration guidelines, such as shortened booster intervals and the National Advisory Committee on Immunization advice recommending the use of a certain vaccine type.
9.54 There were about 169 million vaccine doses paid for from December 2020 to the end of May 2022. Of those, about 124.9 million vaccine doses were delivered to Canada. The agency reported that the majority of these were administered to individuals in Canada. There were close to 32.5 million doses in federal, provincial, and territorial inventories. By the end of our audit period, the majority of these remaining doses still had a shelf life and could be used in booster campaigns or donated; however, most will expire by the end of 2022 if unused (Exhibit 9.3). We estimated that the 32.5 million doses in inventory at the end of May 2022 were worth about $1 billion using the average cost per dose we estimated using publicly available and unclassified information (see paragraph 9.26).
Exhibit 9.3—Most unused doses in Canada will expire by the end of 2022
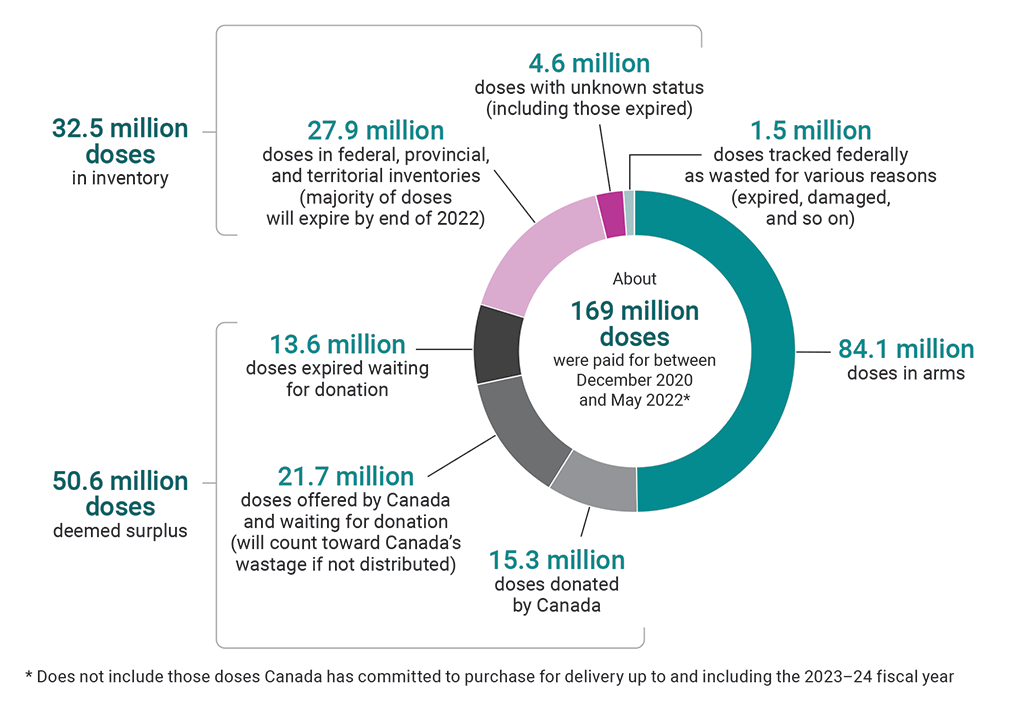
Source: Public Health Agency of Canada
Exhibit 9.3—text version
This pie chart shows the breakdown of the about 169 million vaccine doses that were paid for between December 2020 and May 2022. This number does not include those doses Canada has committed to purchase for delivery up to and including the 2023–24 fiscal year.
About half of the 169 million doses were doses in arms. The remaining doses consisted of the following:
- There were 32.5 million doses in inventory. Of these, 27.9 million were doses in federal, provincial, and territorial inventories (the majority of these doses will expire by the end of 2022), and 4.6 million were doses with unknown status (including those expired).
- There were 50.6 million doses deemed surplus. Of these, 15.3 million were doses donated by Canada, 21.7 million were doses offered by Canada and waiting for donation (these will count toward Canada’s wastage if not distributed), and 13.6 million were doses expired waiting for donation.
- There were 1.5 million doses tracked federally as wasted for various reasons (expired, damaged, and so on).
9.55 Furthermore, we found that the agency was not able to properly track vaccine surplus and wastage once vaccines were delivered to the provinces and territories. A lack of data-sharing agreements with provinces and territories (see paragraph 9.72) affected the agency’s capacity to gather information on the inventory, wastage, and expiry of COVID‑19 vaccine doses. This meant that the agency relied on voluntary reporting by the provinces and territories. Although some provinces and territories consistently reported to the agency, the agency was unable to obtain complete data from most. This meant that the status of these doses was unknown and reduced the agency’s ability to predict supply needs and plan for donations.
9.56 International vaccine donations support Goal 3 of the United Nations’ Sustainable Development Goals, which includes “vaccines for all” as part of one of its targets. The Government of Canada committed to donating the equivalent of 200 million COVID‑19 vaccine doses internationally by the end of 2022. This number includes 50.6 million doses that the agency deemed surplus. We found that as at 31 May 2022, only 15.3 million of these 50.6 million doses had been donated. We also found that, despite a commitment to donate doses with as much shelf life left as possible (ideally 14 to 16 weeks), other countries accepted multiple donations that left them only from 4 to 8 weeks to administer the vaccines before they expired. Officials told us that there are challenges with donations that are outside of Canada’s control, including the willingness of other countries to accept donations offered. Furthermore, many countries are now trying to donate their surplus doses, resulting in a slowdown in demand for donations.
9.57 Recommendation. To minimize further wastage, the Public Health Agency of Canada should draw on the lessons learned from its management of the COVID‑19 vaccine supply and work with other implicated federal organizations and stakeholders to adjust its management of COVID‑19 vaccine surpluses.
The agency’s response. Agreed.
See Recommendations and Responses at the end of this report for detailed responses.
Delays in implementing a vaccine management information technology system
9.58 In our 2021 audit of pandemic preparedness, surveillance, and border control measures, we identified long-standing shortcomings regarding the agency’s information technology infrastructure used for the storage, processing, and analysis of health surveillance data from provinces and territories. For more than 10 years before the COVID‑19 pandemic, the agency identified gaps in its existing infrastructure but had not implemented solutions to improve it.
9.59 Given the infrastructure gap, during the pandemic, the Public Health Agency of Canada needed to rapidly put in place a system to support vaccine management. In January 2021, Deloitte IncorporatedInc. was contracted to develop a national vaccine management information technology system (now called VaccineConnect). Using data from the agency, provinces, and territories, the system was expected to provide timely information on vaccine distribution, coverage, and safety. Some capabilities were to be in place by February 2021 to support order processing, supply chain visibility, inventory tracking, and vaccine coverage tracking.
9.60 We found that, although VaccineConnect was partially functional in June 2021, the agency delayed the development of key system capabilities that would have helped with decision making on wastage (for example, tracking of expiry dates). The contract’s total estimated cost was $59.1 million. We noted that $37.4 million had been spent on VaccineConnect by the end of the audit period.
9.61 Key capabilities of the system were delayed by the agency and not ready in February 2021 as planned. We found that the agency developed workaround procedures and manually tracked expiry dates, wastage, and all the data from the start of the vaccination rollout to June 2021 in spreadsheets. We found that, although some components of the system were functional in June 2021, the agency and the provinces and territories were not using them all, and the agency was continuing to track data using spreadsheets.
9.62 We also found that the agency had limited procedures in place to verify data quality for both its manual tracking and for VaccineConnect. This resulted in gaps in the inventory management data provided and a risk of error. For example, during our review of inventory data, we found that 150,000 doses appeared to have been delivered after they had expired, when in fact, they had not yet expired. Despite the agency’s awareness of the inaccurate expiry date information since October 2021, VaccineConnect was manually updated with the accurate information only close to the end of our audit period. Without the proper verification procedures in place, the agency cannot be assured that the inventory management data being used for future procurement and donation decision making is accurate.
9.63 Recommendation. The Public Health Agency of Canada should complete implementing VaccineConnect. This should include data quality procedures.
The agency’s response. Agreed.
See Recommendations and Responses at the end of this report for detailed responses.
Surveillance of vaccines
Despite data-sharing issues, the Public Health Agency of Canada and Health Canada responded to vaccine surveillance data
9.64 We found that the Public Health Agency of Canada and Health Canada collected and analyzed COVID‑19 vaccine surveillance data to monitor the safety, coverage, and effectiveness of the vaccines. We found that the 2 organizations took actions to respond to the 3 confirmed vaccine safety signalsDefinition 5 based on adverse events reported in Canada, including issuing public information and requiring vaccine label changes. However, we also found that some of the problems regarding sharing of surveillance data that we had raised in 4 previous audits remained. The data-sharing problems affected the agency’s ability to collect disaggregated vaccine coverage data on population characteristics from provinces and territories. These problems also affected the agency’s ability to share information with Health Canada, the vaccine companies, and the World Health Organization.
9.65 The analysis supporting this finding discusses the following topics:
9.66 This finding matters because vaccine surveillance data provides information to the Public Health Agency of Canada and Health Canada on public health and regulatory actions necessary for ensuring the continued safe and effective use of COVID‑19 vaccines. Responding to this information in a timely way can raise the awareness of potential risks and help prevent serious adverse events from affecting more people.
9.67 Health surveillance is the continuing and systematic collection, analysis, and dissemination of information to enable decision making and action to protect the health of populations. The Public Health Agency of Canada and Health Canada obtain and use surveillance information from several sources, including other countries. International health surveillance is particularly important if vaccine use starts earlier in other countries or if rare adverse events might be detected only in countries with a larger population than Canada’s.
9.68 There are 3 areas of responsibility regarding COVID‑19 vaccine surveillance: safety, coverage, and effectiveness. The Public Health Agency of Canada and Health Canada have distinct and complementary roles for monitoring safety and effectiveness. The agency is responsible for monitoring coverage.
9.69 Both the agency and the department had surveillance systems in place to monitor the safety of all vaccines approved for use in Canada prior to the pandemic, and these systems were used for COVID‑19 vaccine surveillance. Provinces and territories voluntarily submit reports of adverse events following immunizationDefinition 6 to the agency’s Canadian Adverse Events Following Immunization Surveillance System. Reports are also submitted to the department’s Canada Vigilance Program, primarily by vaccine companies, which are required to continue to carry out safety monitoring once vaccines have been introduced for use in Canada. Health professionals and consumers can also submit information voluntarily to the program. Adverse events are monitored and analyzed by the agency and the department so that vaccines remain safe and that appropriate actions are taken when a vaccine safety signal is identified.
Response to information on vaccine safety
9.70 We found that the Public Health Agency of Canada and Health Canada monitored the safety and effectiveness of COVID‑19 vaccines by obtaining and analyzing surveillance data, including post-market studies, safety reports, and adverse events following immunization reported internationally and domestically. Since the beginning of the COVID‑19 mass vaccination campaign in Canada, the Public Health Agency of Canada and Health Canada confirmed 3 vaccine safety signals based on adverse events reported in Canada: thrombosis with thrombocytopenia syndrome, myocarditis and pericarditis, and Guillain-Barré Syndrome. These safety signals were initially detected internationally.
9.71 We found that following the international detection of these signals, Health Canada completed initial safety assessments for each of the 3 confirmed signals in an average of approximately 14 days, which is faster than its non-pandemic performance target of 130 days. We also found that
- as part of its response to these safety signals, the Public Health Agency of Canada and Health Canada issued public information to inform health-care providers and the population at large
- after advising the relevant vaccine companies to revise their vaccine labels, Health Canada completed its review of the revisions submitted by the vaccine companies faster than the non-pandemic service standard (from 165 days to an average of approximately 7 days for each of the 3 confirmed signals)
- the National Advisory Committee on Immunization issued recommendations for provinces and territories about the use of the implicated vaccines
Timely response by the agency and the department to these confirmed safety signals was necessary to inform health-care professionals and Canadians of potential serious adverse events and to support informed decision making around the safe use of COVID‑19 vaccines.
Data-sharing problems remain
9.72 We found that some of the problems regarding the sharing of surveillance data that had been raised in several previous audits remained. We raised issues in 1999, 2002, and 2008 and, most recently, in the 2021 audit of pandemic preparedness, surveillance, and border control measures. In the 2021 audit, we reported that the agency did not have regulations for the collection, use, and disclosure of public health information and that it had not finalized with its provincial and territorial partners which elements of health information should be provided, to whom, and in what format. Also in the 2021 audit, we found that supporting information technology infrastructure had not been developed. In the current audit, we found that those long-standing issues affected the effectiveness of the Public Health Agency of Canada’s sharing of detailed case-level safety surveillance data with Health Canada, as well as with the World Health Organization and vaccine companies. These issues also affected the agency’s ability to collect disaggregated vaccine coverage data on population characteristics (for example, ethnicity).
9.73 The Public Health Agency of Canada and Health Canada had different surveillance systems for monitoring vaccine safety already in place and used them to obtain and analyze surveillance data on COVID‑19 vaccines. However, we found that the agency could share information received from provinces and territories through the Canadian Adverse Events Following Immunization Surveillance System (CAEFISS) with others only when provinces and territories provided specific consent to do so. In early April 2021, the agency requested permission from provinces and territories to make specific CAEFISS data elements publicly available to all partners, including Health Canada, the World Health Organization, and vaccine companies, to enhance timely safety signal detection. Permission was not received from all provinces and territories, with some citing privacy concerns. In addition, in July 2021, Health Canada sent a request to the agency to have access to CAEFISS to “facilitate regulatory review and decision making based on comprehensive and up-to-date safety information” for COVID‑19 vaccines. By the end of our audit period, the agency had not received permission from provinces and territories to provide Health Canada access to CAEFISS.
9.74 We also noted that, according to the agency, Canada is the only Group of 7G7 country that does not follow World Health Organization guidance that countries share with the organization detailed case-level data on COVID‑19 adverse events following immunization. The agency shared summary-level data with the organization.
9.75 In addition, Health Canada requires vaccine companies to carry out safety monitoring once vaccines have been authorized for use. Companies cannot entirely fulfill this requirement when they do not have access to the necessary data on adverse events. We found that in 2 separate instances in 2021, 2 vaccine companies learned of Canadian reports of adverse events following immunization through the media and subsequently requested the underlying data from the agency on an urgent basis. The Public Health Agency of Canada had to seek provincial and territorial consent to provide the data. We found that in both cases, it took approximately 3 months to obtain permission and to provide all of the information to the vaccine companies.
9.76 The authorization of a Canadian COVID‑19 vaccine, and the government’s renewed support to manufacture novel vaccines domestically, which increases the likelihood of other vaccines being authorized in Canada first, may reduce the Public Health Agency of Canada’s and Health Canada’s ability to leverage international sources for vaccine surveillance information. This also increases the importance of Canada sharing pertinent surveillance information domestically and with its international partners.
9.77 We found that, to monitor coverage, the agency established the Canadian COVID‑19 Vaccination Coverage Surveillance System at the start of the pandemic. The agency initially manually compiled data submitted voluntarily by provinces and territories, but by June 2021, it was processing this data electronically. However, our review of the data showed that some population characteristics, such as ethnicity or Indigenous status, were not included, because provinces and territories do not always collect or share that information. In our view, without this type of disaggregated data, the agency could have less information to target programs or communications to groups that may be at higher risk.
9.78 Since October 2020, the Public Health Agency of Canada and Health Canada have led work with partners, including provinces, territories, and Indigenous organizations, on the Pan-Canadian Health Data Strategy. The strategy’s aim is to advance efforts to improve health data collection, sharing, and usage. In a 2021 report, the Chief Public Health Officer of Canada noted that the strategy was not expected to be implemented prior to 2030 and encouraged the acceleration of its implementation.
9.79 Recommendation. Given the urgency and importance of improving timely access to quality data among health partners, the Public Health Agency of Canada and Health Canada should expedite their work with provinces and territories to implement the Pan-Canadian Health Data Strategy.
Response of each entity. Agreed.
See Recommendations and Responses at the end of this report for detailed responses.
9.80 Recommendation. The Public Health Agency of Canada, in collaboration with Health Canada and the provinces and territories, should resolve barriers to
- better share vaccine surveillance information among themselves
- provide access to the Canadian Adverse Events Following Immunization Surveillance System to Health Canada
- provide surveillance data, including case-level details as needed, to the World Health Organization and vaccine companies in a timely manner
The agency’s response. Agreed.
See Recommendations and Responses at the end of this report for detailed responses.
Conclusion
9.81 We concluded that Public Services and Procurement Canada provided efficient procurement support and secured a sufficient number of COVID‑19 vaccine doses to vaccinate Canada’s population.
9.82 We also concluded that the Public Health Agency of Canada and Health Canada efficiently provided access to COVID‑19 vaccines. Health Canada expedited its regulatory process to authorize the vaccines, and the Public Health Agency of Canada distributed vaccine doses to the provinces and territories in a timely manner. However, the agency was unsuccessful in minimizing vaccine wastage.
9.83 Lastly, we concluded that the Public Health Agency of Canada and Health Canada were timely in responding to the 3 confirmed vaccine safety signals based on adverse events reported in Canada. However, long-standing issues related to data sharing identified in past audits affected the effectiveness of the agency’s sharing of detailed case-level safety surveillance data with Health Canada, as well as with the World Health Organization and vaccine companies.
About the Audit
This independent assurance report was prepared by the Office of the Auditor General of Canada on COVID‑19 vaccines. Our responsibility was to provide objective information, advice, and assurance to assist Parliament in its scrutiny of the government’s management of resources and programs, and to conclude on whether the selected federal organizations involved in COVID‑19 vaccines complied in all significant respects with the applicable criteria.
All work in this audit was performed to a reasonable level of assurance in accordance with the Canadian Standard on Assurance Engagements (CSAE) 3001—Direct Engagements, set out by the Chartered Professional Accountants of Canada (CPA Canada) in the CPA Canada Handbook—Assurance.
The Office of the Auditor General of Canada applies the Canadian Standard on Quality Control 1 and, accordingly, maintains a comprehensive system of quality control, including documented policies and procedures regarding compliance with ethical requirements, professional standards, and applicable legal and regulatory requirements.
In conducting the audit work, we complied with the independence and other ethical requirements of the relevant rules of professional conduct applicable to the practice of public accounting in Canada, which are founded on fundamental principles of integrity, objectivity, professional competence and due care, confidentiality, and professional behaviour.
In accordance with our regular audit process, we obtained the following from entity management:
- confirmation of management’s responsibility for the subject under audit
- acknowledgement of the suitability of the criteria used in the audit
- confirmation that all known information that has been requested, or that could affect the findings or audit conclusion, has been provided
- confirmation that the audit report is factually accurate
Audit objective
The objective of this audit was to determine whether
- Public Services and Procurement Canada provided adequate procurement support to secure vaccines
- the Public Health Agency of Canada and Health Canada efficiently provided access to COVID‑19 vaccines
- the Public Health Agency of Canada and Health Canada’s surveillance of the COVID‑19 vaccine was effective and timely
Scope and approach
The audit scope included the 3 main federal organizations involved in implementing Canada’s COVID‑19 Immunization Plan: Saving Lives and Livelihoods—the Public Health Agency of Canada, Health Canada, and Public Services and Procurement Canada.
We interviewed officials involved in the different areas under our scope. We also interviewed officials at National Research Council Canada who were responsible for the COVID‑19 Vaccine Task Force secretariat to obtain documentation.
We did not audit the quality of science-based decisions or the scientific judgments in any of our audit work.
With regard to our audit work on procurement, we examined the actions taken by Public Services and Procurement Canada to procure a sufficient number of vaccines. We reviewed documentation from the 7 advance purchase agreements. We are limited in what we can report because of the confidentiality clauses in the agreements. To estimate an average cost per dose, we used unclassified information and information available in the Public Accounts of Canada. The Public Accounts of Canada aggregated amounts disbursed for vaccines, including advance payments, and therapeutics. The amount disbursed is an aggregate because there is no publicly available information that discloses the exact amount paid to companies for COVID‑19 vaccines. This is in order to respect the confidentiality clauses in the agreements. The estimated average cost is a reasonable approximation of the actual average cost per dose.
We reviewed key documentation prepared by Health Canada to authorize different COVID‑19 vaccines to see if the steps in the expedited process had been followed. We also reviewed documents related to the quality framework in place.
We examined documents and analyzed data related to
- the allocation and distribution of vaccines (from the Public Health Agency of Canada)
- vaccine surveillance (from the Public Health Agency of Canada and Health Canada)
In this audit report, we noted that there were limited procedures in place at the Public Health Agency of Canada to verify the quality of its inventory management data. Although we are confident in our analyses and observations in this report, they were based on the agency’s data that was available to us.
This audit contributed to Canada’s actions in relation to the United Nations’ Sustainable Development Goal 3—Good health and well-being.
Criteria
We used the following criteria to determine whether
- Public Services and Procurement Canada provided adequate procurement support to secure vaccines
- the Public Health Agency of Canada and Health Canada efficiently provided access to COVID‑19 vaccines
- the Public Health Agency of Canada and Health Canada’s surveillance of the COVID‑19 vaccine was effective and timely
| Criteria | Sources |
|---|---|
|
The Public Health Agency of Canada identifies, in an efficient manner and based on scientific advice available at the time, the specifications required to procure COVID‑19 vaccines. |
|
|
Public Services and Procurement Canada’s COVID‑19 vaccine procurement is conducted in an efficient manner and with due diligence to meet the specifications identified by the Public Health Agency of Canada |
|
|
Health Canada follows the authorization process established for the COVID‑19 vaccines. |
|
|
The Public Health Agency of Canada efficiently and equitably allocates COVID‑19 vaccines. |
|
|
The Public Health Agency of Canada efficiently distributes COVID‑19 vaccines. |
|
|
The Public Health Agency of Canada and Health Canada collect and analyze accurate and timely COVID‑19 vaccine surveillance data in order to monitor the safety, coverage, and effectiveness of COVID‑19 vaccines and to take public health actions and regulatory actions if required. |
|
Period covered by the audit
The audit covered the period from 1 January 2020 to 31 May 2022. This is the period to which the audit conclusion applies. However, to gain a more complete understanding of the subject matter of the audit, we also examined certain matters that preceded the start date of this period.
Date of the report
We obtained sufficient and appropriate audit evidence on which to base our conclusion on 29 September 2022, in Ottawa, Canada.
Audit team
This audit was completed by a multidisciplinary team from across the Office of the Auditor General of Canada led by Susan Gomez, Principal. The principal has overall responsibility for audit quality, including conducting the audit in accordance with professional standards, applicable legal and regulatory requirements, and the office’s policies and system of quality management.
Recommendations and Responses
In the following table, the paragraph number preceding the recommendation indicates the location of the recommendation in the report.
| Recommendation | Response |
|---|---|
|
9.57 To minimize further wastage, the Public Health Agency of Canada should draw on the lessons learned from its management of the COVID‑19 vaccine supply and work with other implicated federal organizations and stakeholders to adjust its management of COVID‑19 vaccine surpluses. |
Agreed. From the onset of the pandemic the Government of Canada’s primary objective has been to ensure that Canada has timely access to the most effective vaccines to protect the health and safety of Canadians. Canada’s COVID‑19 vaccine supply plans have evolved throughout the pandemic, informed by emerging scientific evidence, timing of regulatory approvals, product availability, National Advisory Committee on Immunization guidance, and federal/provincial/territorial needs. The Public Health Agency of Canada will review lessons learned and collaborate with other implicated departments and stakeholders to optimize COVID‑19 vaccine supply management and reduce COVID‑19 vaccine surpluses and wastage throughout the duration of the contracts. These continued efforts will include:
The agency will also draw on the lessons learned from COVID‑19 to help inform vaccine supply planning for future pandemics (December 2024). |
|
9.63 The Public Health Agency of Canada should complete implementing VaccineConnect. This should include data quality procedures. |
Agreed. The Public Health Agency of Canada is actively working to advance the implementation and data quality procedures of the three modules of VaccineConnect; namely, the Intelligent Supply Chain, the Immunization Information System and the Immunization Program Management. The agency will continue to actively engage jurisdictional partners on identification of service gaps and needs to support future integration of the systems. Building on investments to date in the Intelligent Supply Chain module of VaccineConnect, the agency will work closely with other federal departments, as well as provincial and territorial partners to support supply chain management and distribution of vaccines. The Immunization Information System will replace the Canadian Adverse Events Following Immunization Surveillance System. The agency will test and validate the cloud Canadian Adverse Events Following Immunization Surveillance System in order to prepare for the anticipated minimum viable product launch in fall 2022. The development of the Immunization Program Management module is complete, and it was leveraged by a number of jurisdictions during the deployment of the COVID‑19 vaccination campaign. |
|
9.79 Given the urgency and importance of improving timely access to quality data among health partners, the Public Health Agency of Canada and Health Canada should expedite their work with provinces and territories to implement the Pan-Canadian Health Data Strategy. |
The Public Health Agency of Canada’s response. Agreed. The Public Health Agency of Canada created the Corporate Data and Surveillance Branch in October 2020 to support its commitment to continue improving health data collection, sharing and use as their response to recommendation 8.66 in the 2021 Reports of the Auditor General of Canada to the Parliament of Canada, Report 8—Pandemic Preparedness, Surveillance, and Border Control Measures. The agency and Health Canada have been working with provinces and territories to co-develop the Pan-Canadian Health Data Strategy. This strategy will address the long-standing issues affecting Canada’s ability to collect, share, access and use health data. Implementation will be guided by aligned policies and frameworks with an integrated workplan. A review is planned for every three years. The pace of implementation will respect individual jurisdictions’ capacity, readiness, and seek opportunities to accelerate implementation through collaboration. Health Canada’s response. Agreed. Health Canada will continue to collaborate with the Public Health Agency of Canada to advance the development and implementation of the Pan-Canadian Health Data Strategy, working with provinces and territories. |
|
9.80 The Public Health Agency of Canada, in collaboration with Health Canada and the provinces and territories, should resolve barriers to
|
Agreed. The Public Health Agency of Canada understands that information sharing is an important component of Canada’s vaccine safety surveillance system, which is a collaboration between provinces and territories, the agency, Health Canada, and vaccine manufacturers, and will continue to advance better information sharing with its partners. The agency is leading consultations with provincial and territorial partners on a proposal to provide Health Canada with access to the Canadian Adverse Events Following Immunization Surveillance System (CAEFISS). CAEFISS receives reports from all provincial and territorial public health authorities and from some federal departments. Some provinces and territories have put conditions on the access to and use of the health information they provide to the agency. The agency will continue to share CAEFISS data in aggregate form with the World Health Organization on a regular schedule, and on an as-needed basis with vaccine companies. The agency will engage with provinces and territories in an effort to allow it to release more granular data to the World Health Organization and vaccine companies, as needed, while recognizing the importance of protecting patient confidentiality, respecting privacy laws, and supporting accurate interpretation of the data. |

